- Record: found
- Abstract: found
- Article: found
Hypoxic postconditioning-induced neuroprotection increases neuronal autophagy via activation of the SIRT1/FoxO1 signaling pathway in rats with global cerebral ischemia
Read this article at
Abstract
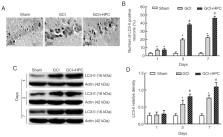
Hypoxic postconditioning (HPC) has been reported to be a beneficial and promising treatment for global cerebral ischemia (GCI). However, its neuroprotective mechanism remains unclear. The aim of the present study was to determine whether the protective effects of HPC in a rat model of GCI were due to the upregulation of autophagy via the silent information regulator transcript-1 (SIRT1)/Forkhead box protein 1 (FoxO1) pathway. Morris water maze test revealed that HPC attenuated cognitive damage in GCI rats. HPC also significantly increased the levels of the autophagy-related protein LC3-II, SIRT1 and FoxO1 compared with those in the GCI group. However, the HPC-induced LC3-II upregulation was blocked by the SIRT1 inhibitor EX527. These results suggested that the beneficial effects of HPC on GCI rats were due to the upregulation of ischemiainduced autophagy and involved the SIRT1/FoxO1 signaling pathway.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Autophagy: process and function.
- Record: found
- Abstract: found
- Article: not found
Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials.
- Record: found
- Abstract: found
- Article: not found