- Record: found
- Abstract: found
- Article: found
The inwardly rectifying K + channel KIR7.1 controls uterine excitability throughout pregnancy
Read this article at
Abstract
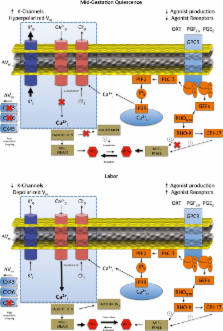
Abnormal uterine activity in pregnancy causes a range of important clinical disorders, including preterm birth, dysfunctional labour and post-partum haemorrhage. Uterine contractile patterns are controlled by the generation of complex electrical signals at the myometrial smooth muscle plasma membrane. To identify novel targets to treat conditions associated with uterine dysfunction, we undertook a genome-wide screen of potassium channels that are enriched in myometrial smooth muscle. Computational modelling identified Kir7.1 as potentially important in regulating uterine excitability during pregnancy. We demonstrate Kir7.1 current hyper-polarizes uterine myocytes and promotes quiescence during gestation. Labour is associated with a decline, but not loss, of Kir7.1 expression. Knockdown of Kir7.1 by lentiviral expression of miRNA was sufficient to increase uterine contractile force and duration significantly. Conversely, overexpression of Kir7.1 inhibited uterine contractility. Finally, we demonstrate that the Kir7.1 inhibitor VU590 as well as novel derivative compounds induces profound, long-lasting contractions in mouse and human myometrium; the activity of these inhibitors exceeds that of other uterotonic drugs. We conclude Kir7.1 regulates the transition from quiescence to contractions in the pregnant uterus and may be a target for therapies to control uterine contractility.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
ATP-sensitive potassium channelopathies: focus on insulin secretion.
- Record: found
- Abstract: found
- Article: not found
A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene.
- Record: found
- Abstract: found
- Article: not found