- Record: found
- Abstract: found
- Article: found
Heterogeneity and plasticity of epithelial–mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms
Read this article at
Abstract
Epithelial–mesenchymal transition (EMT) or mesenchymal–epithelial transition (MET) plays critical roles in cancer metastasis. Recent studies, especially those based on single‐cell sequencing, have revealed that EMT is not a binary process, but a heterogeneous and dynamic disposition with intermediary or partial EMT states. Multiple double‐negative feedback loops involved by EMT‐related transcription factors (EMT‐TFs) have been identified. These feedback loops between EMT drivers and MET drivers finely regulate the EMT transition state of the cell. In this review, the general characteristics, biomarkers and molecular mechanisms of different EMT transition states were summarized. We additionally discussed the direct and indirect roles of EMT transition state in tumour metastasis. More importantly, this article provides direct evidence that the heterogeneity of EMT is closely related to the poor prognosis in gastric cancer. Notably, a seesaw model was proposed to explain how tumour cells regulate themselves to remain in specific EMT transition states, including epithelial state, hybrid/intermediate state and mesenchymal state. Additionally, this article also provides a review of the current status, limitations and future perspectives of EMT signalling in clinical applications.
Abstract
-
The general biomarkers in EMT signalling pathway were updated.
-
Cellular EMT transition state is continuous, including epithelial state, diverse hybrid states (p‐EMT states) and mesenchymal state. Tumour cells in p‐EMT state may play a role in hematogenous metastasis due to their extreme plasticity.
-
We propose a seesaw model between EMT drivers and MET drivers to explain how cells regulate their own EMT transformation state. The expression of EMT drivers and MET drivers is finely regulated by the double negative feedback loop in EMT signalling.
-
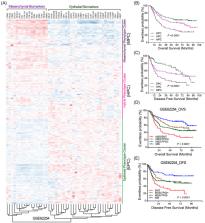
Tumour heterogeneity should be partly attributed to the EMT plasticity of tumour cells. The inter‐tumour heterogeneity is clinical associated with poor prognosis in gastric cancer.
-
EMT signalling plays crucial roles in cancer metastasis, stemness, chemoresistance and immune suppression. Targeting EMT signalling is a promising anti‐tumour strategy that kills numerous birds with one stone.
Related collections
Most cited references161
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms of epithelial-mesenchymal transition.
- Record: found
- Abstract: found
- Article: not found
EMT Transition States during Tumor Progression and Metastasis
- Record: found
- Abstract: found
- Article: found
