- Record: found
- Abstract: found
- Article: found
Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt β-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4
Read this article at
Abstract
Background: Identification of breast cancer stem cells as the chemo-resistant and tumor-initiating population represents an important milestone in approaching anticancer therapies. Targeting this minor subpopulation of chemo- and radio-resistant stem-like cells, termed as the cancer stem cells (CSCs) and their eradication could significantly enhance clinical outcomes. Most of the presently administered chemotherapeutics target the tumor bulk but are ineffective against the CSCs. We report here that diosgenin (DG), a naturally occurring steroidal saponin, could effectively inhibit CSCs from three breast cancer cell lines, MCF7, T47D and MDA-MB-231, by inducing apoptosis and inhibiting the CSC associated phenotypes.
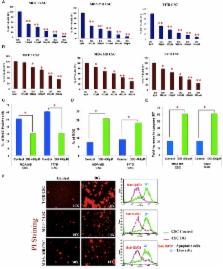
Methods: CSCs were enriched in these cells lines, characterized for CSC traits by immunocytochemistry and flow cytometry. Proliferation and apoptosis assays were performed in these breast CSCs in the presence of DG to obtain the inhibitory concentration. Apoptosis was confirmed with gene expression analysis, Western blotting and propidium iodide staining. TCF-LEF reporter assay, sFRP overexpression and RNAi silencing studies were performed to study regulation of the Wnt pathway. Statistical significance was evaluated by a two-sided Student’s t-test.
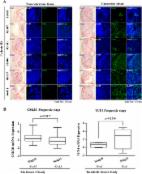
Results: Using the TCF-LEF reporter system, we show the effect of DG on CSCs is predominantly through the network regulating CSC self renewal, the Wnt β-catenin pathway. Specifically, the Wnt antagonist, the secreted frizzled related protein 4, (sFRP4), had a defining role in the action of DG. Gain-of-function of sFRP4 in CSCs could improve the response to DG wherein CSC mediators were inhibited, β-catenin was down regulated and the effectors of epithelial to mesenchymal transition and pro-invasive markers were repressed. Conversely, the loss-of-function of sFRP4 had a reverse effect on the CSC population which therein became enriched, their response to DG treatment was modest, β-catenin levels increased, GSK3β expression decreased and the expression of epithelial markers of CSC was completely abrogated.
Conclusion: These findings demonstrate the effect of DG on inhibiting the resilient breast CSCs which could provide a benchmark for the development of DG-based therapies in breast cancer treatment.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: found
Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype
- Record: found
- Abstract: found
- Article: not found
Targeting breast stem cells with the cancer preventive compounds curcumin and piperine.
- Record: found
- Abstract: found
- Article: not found