- Record: found
- Abstract: found
- Article: found
Chemical signature of colorectal cancer: case–control study for profiling the breath print
Read this article at
Abstract
Background
Effective screening for colorectal cancer can reduce mortality by early detection of tumours and colonic polyps. An altered pattern of volatile organic compounds (VOCs) in exhaled breath has been proposed as a potential non‐invasive diagnostic tool for detection of cancer. The aim of this study was to evaluate the reliability of breath‐testing for colorectal cancer screening and early diagnosis using an advanced breath sampler.
Methods
The exhaled breath of patients with colorectal cancer and non‐cancer controls with negative findings on colonoscopy was collected using the ReCIVA® Breath Sampler. This portable device is able to capture the alveolar breath fraction without environmental contamination. VOCs were desorbed thermally and analysed by gas chromatography–mass spectrometry. The discriminatory ability of VOCs in detecting colorectal cancer was evaluated by receiver operating characteristic (ROC) curve analysis for each VOC, followed by cross‐validation by the leave‐one‐out method, and by applying stepwise logistic regression analysis.
Results
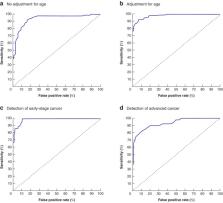
The study included 83 patients with colorectal cancer and 90 non‐cancer controls. Fourteen VOCs were found to have significant discriminatory ability in detecting patients with colorectal cancer. The model with the diagnosis of cancer versus no cancer resulted in a statistically significant likelihood of discrimination of 173·45 ( P < 0·001), with an area under the ROC curve of 0·979. Cross‐validation of the model resulted in a true predictive value for colorectal cancer of 93 per cent overall. Reliability of the breath analysis was maintained irrespective of cancer stage.
Conclusion
This study demonstrated that analysis of exhaled VOCs can discriminate patients with colorectal cancer from those without. This finding may eventually lead to the creation of a smart online sensory device, capable of providing a binary answer (cancer/no cancer) and directing to further screening.
Abstract
Available screening methods for colorectal cancer have poor reliability and low patient compliance. Cancer cells produce altered metabolites, which are transported in the bloodstream and released in the alveoli; these volatile organic compounds (VOCs) can be detected in exhaled breath. This study used a new‐generation breath sampler, capable of selecting the alveolar fraction of the breath and preventing environmental contamination. A pattern of 14 VOCs was able to discriminate patients with colorectal cancer from healthy controls, with a true predictive value of 93 per cent, irrespective of cancer stage.
Breath test signature in colorectal cancer
Translated abstract
Un cribaje efectivo del cáncer colorrectal ( colorectal cáncer, CRC) puede reducir la mortalidad mediante la detección precoz de cáncer/pólipos del colon. La identificación de un patrón de compuestos volátiles orgánicos ( volatile organic compounds, VOCs) en el aire espirado se ha propuesto como un procedimiento potencial de diagnóstico no invasivo para la detección del cáncer. El objetivo de este estudio fue evaluar la factibilidad del test de la respiración para el cribaje del CRC y diagnóstico precoz empleando un equipo avanzado de muestreo del aliento.
Se recogieron muestras de aire espirado de 83 pacientes con CRC y de 90 controles sin cáncer con colonoscopia negativa empleando el ReCIVA Breath Sampler©. Este equipo portátil es capaz de capturar la fracción de aire alveolar espirada ausente de contaminación ambiental. Los VOCs fueron aislados térmicamente y analizados mediante cromatografía de gases acoplada a espectrometría de masas. La capacidad discriminatoria de los VOCs para detectar pacientes con CCR se evaluó mediante un análisis de la curva ROC para cada VOC seguida de validación cruzada mediante el método ir eliminando paso a paso cada uno de los VOCs en un modelo de regresión logística.
Se observó que 14 VOCs tenían habilidad discriminatoria significativa para la detección de pacientes con CRC. El modelo con el diagnóstico de cáncer versus no cáncer mostró una probabilidad estadísticamente significativa de 151,03 ( P < 0,0001) con un área bajo la curva ( area under the curve, AUC) de 0,963. En la validación cruzada del modelo se obtuvo un valor global predictivo verdadero para el CRC del 92,5%. La fiabilidad del análisis del aire espirado se mantuvo con independencia del estadio del cáncer.
Este estudio ha demostrado que el análisis de los VOCs en el aire espirado puede discriminar pacientes con CRC de pacientes sin cáncer. Este hallazgo podría ser de ayuda para diceñar un dispositivo sensorial inteligente en línea, capaz de proporcionar una respuesta binaria (cáncer/NO cáncer) y asimismo contribuir a la indicación de una futura colonoscopia.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors

- Record: found
- Abstract: found
- Article: found
Dysbiosis of gut microbiota in promoting the development of colorectal cancer

- Record: found
- Abstract: found
- Article: found
