- Record: found
- Abstract: found
- Article: found
World Café approach: exploring the future vision of oral anticoagulants for patients with atrial fibrillation (AF) in Ireland
Abstract
Objectives
To explore and reflect on the current anticoagulation therapy offered to patients with atrial fibrillation (AF), potential challenges and the future vision for oral anticoagulants for patients with AF and healthcare professionals in Ireland.
Participants
Nine participants from academic, clinical and health backgrounds attended the focus group together with a facilitator.
Results
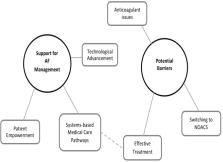
Enhanced patient empowerment; more effective use of technology and developing system-based medical care pathways would provide improved supports for AF management. The challenges in providing these include cost and access issues, the doctor–patient relationship and the provision of education. While consensus for developing evidence-based pathways to maximise efficiency and effectiveness of AF treatment was evident, it would require a shared vision between stakeholders of integrated care. The benefits of embracing technological advances for clinicians and patients were evident; however, clinicians indicate this can increase pressure on already stretched resources; coupled with institutional barriers (including scarce resources) arising from the complex nature of anticoagulation for patients with AF, which emerged strongly. Including the unpredictable nature of warfarin, hidden costs associated with monitoring, adverse clinical effects, different patient cohorts (including those prescribed anticoagulant for the first time vs those switching from warfarin to a new oral anticoagulant (NOAC)), non-adherence concerns and undesirable impacts on patients’ daily lives.
Conclusions
While anticoagulation therapy for patients with AF using NOACs has been widely adopted and is diffusing into routine practice, significant operationalisation issues and barriers to effective treatment/management persist. The reflections reported in this study are a catalyst for future discussion and research.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Using thematic analysis in psychology
- Record: found
- Abstract: found
- Article: not found
Standards for reporting qualitative research: a synthesis of recommendations.
- Record: found
- Abstract: not found
- Article: not found