- Record: found
- Abstract: found
- Article: found
Midbrain circuit regulation of individual alcohol drinking behaviors in mice
Read this article at
Abstract
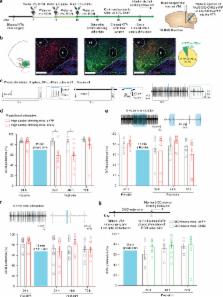
Alcohol-use disorder (AUD) is the most prevalent substance-use disorder worldwide. There is substantial individual variability in alcohol drinking behaviors in the population, the neural circuit mechanisms of which remain elusive. Utilizing in vivo electrophysiological techniques, we find that low alcohol drinking (LAD) mice have dramatically higher ventral tegmental area (VTA) dopamine neuron firing and burst activity. Unexpectedly, VTA dopamine neuron activity in high alcohol drinking (HAD) mice does not differ from alcohol naive mice. Optogenetically enhancing VTA dopamine neuron burst activity in HAD mice decreases alcohol drinking behaviors. Circuit-specific recordings reveal that spontaneous activity of nucleus accumbens-projecting VTA (VTA-NAc) neurons is selectively higher in LAD mice. Specifically activating this projection is sufficient to reduce alcohol consumption in HAD mice. Furthermore, we uncover ionic and cellular mechanisms that suggest unique neuroadaptations between the alcohol drinking groups. Together, these data identify a neural circuit responsible for individual alcohol drinking behaviors.
Abstract
Mice exposed to a two-bottle alcohol choice paradigm can be divided into high and low drinking groups. Here, the authors show that stimulating VTA neurons to induce higher phasic activity patterns that are observed in low alcohol drinking mice, suppresses alcohol drinking in mice that are high alcohol drinking.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Neurobiologic Advances from the Brain Disease Model of Addiction.
- Record: found
- Abstract: found
- Article: not found
Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons
- Record: found
- Abstract: found
- Article: not found