- Record: found
- Abstract: found
- Article: found
Cilium structure, assembly, and disassembly regulated by the cytoskeleton
Read this article at
Abstract
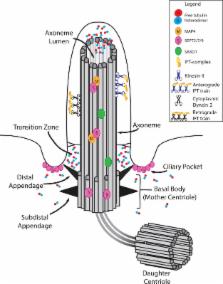
The cilium, once considered a vestigial structure, is a conserved, microtubule-based organelle critical for transducing extracellular chemical and mechanical signals that control cell polarity, differentiation, and proliferation. The cilium undergoes cycles of assembly and disassembly that are controlled by complex inter-relationships with the cytoskeleton. Microtubules form the core of the cilium, the axoneme, and are regulated by post-translational modifications, associated proteins, and microtubule dynamics. Although actin and septin cytoskeletons are not major components of the axoneme, they also regulate cilium organization and assembly state. Here, we discuss recent advances on how these different cytoskeletal systems affect cilium function, structure, and organization.
Related collections
Most cited references151
- Record: found
- Abstract: found
- Article: not found
HDAC6 modulates cell motility by altering the acetylation level of cortactin.
- Record: found
- Abstract: found
- Article: not found
Cep164, a novel centriole appendage protein required for primary cilium formation
- Record: found
- Abstract: found
- Article: not found