- Record: found
- Abstract: found
- Article: found
Plasmid Replicons for the Production of Pharmaceutical-Grade pDNA, Proteins and Antigens by Lactococcus lactis Cell Factories
Read this article at
Abstract
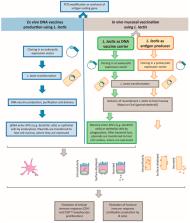
The Lactococcus lactis bacterium found in different natural environments is traditionally associated with the fermented food industry. But recently, its applications have been spreading to the pharmaceutical industry, which has exploited its probiotic characteristics and is moving towards its use as cell factories for the production of added-value recombinant proteins and plasmid DNA (pDNA) for DNA vaccination, as a safer and industrially profitable alternative to the traditional Escherichia coli host. Additionally, due to its food-grade and generally recognized safe status, there have been an increasing number of studies about its use in live mucosal vaccination. In this review, we critically systematize the plasmid replicons available for the production of pharmaceutical-grade pDNA and recombinant proteins by L. lactis. A plasmid vector is an easily customized component when the goal is to engineer bacteria in order to produce a heterologous compound in industrially significant amounts, as an alternative to genomic DNA modifications. The additional burden to the cell depends on plasmid copy number and on the expression level, targeting location and type of protein expressed. For live mucosal vaccination applications, besides the presence of the necessary regulatory sequences, it is imperative that cells produce the antigen of interest in sufficient yields. The cell wall anchored antigens had shown more promising results in live mucosal vaccination studies, when compared with intracellular or secreted antigens. On the other side, engineering L. lactis to express membrane proteins, especially if they have a eukaryotic background, increases the overall cellular burden. The different alternative replicons for live mucosal vaccination, using L. lactis as the DNA vaccine carrier or the antigen producer, are critically reviewed, as a starting platform to choose or engineer the best vector for each application.
Related collections
Most cited references194

- Record: found
- Abstract: found
- Article: found
DNA Vaccines—How Far From Clinical Use?
- Record: found
- Abstract: found
- Article: not found
Synthetic DNA delivery systems.
- Record: found
- Abstract: found
- Article: not found