- Record: found
- Abstract: found
- Article: found
Toxoplasma gondii in small exotic felids from zoos in Europe and the Middle East: serological prevalence and risk factors

Read this article at
Abstract
Background
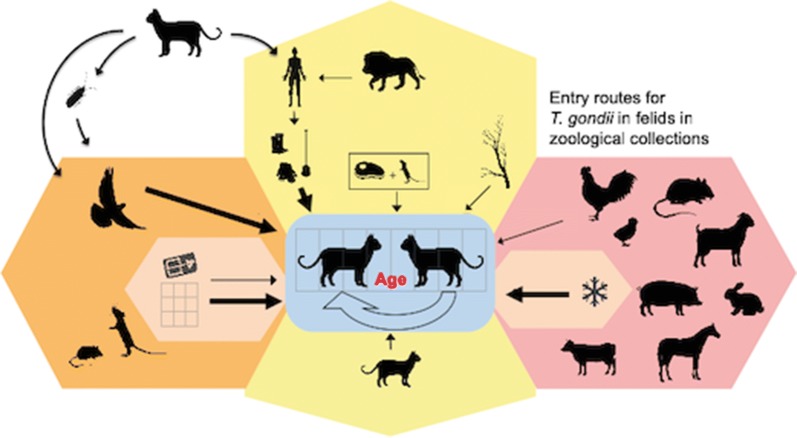
Toxoplasma gondii infections and cases of clinical toxoplasmosis have been recorded in zoo animals. Wild felids in human care can serve as definitive hosts that shed oocysts, but also as intermediate hosts for the parasite. Some felid species, such as the Pallas’s cat ( Otocolobus manul) or sand cat ( Felis margarita), may suffer from clinically apparent toxoplasmosis. In the present study, our main aim was to assess risk factors for T. gondii infections in small exotic felids.
Methods
A seroepidemiological study was conducted using the reduviid bug Dipetalogaster maxima for blood sample collection, a method previously evaluated on domestic cats. A total of 336 samples from 17 felid species were collected in 51 institutions, 48 of which were within Europe and the remaining three in the Middle East (United Arabic Emirates and Qatar). These samples were analyzed for T. gondii antibodies by immunoblotting and an immunofluorescent antibody test. Potential risk factors in zoos for seropositivity regarding T. gondii among members of the European Association of Zoos and Aquaria (EAZA) were evaluated using a questionnaire and individual data from the Zoological Information Management System (ZIMS).
Results
The sampled felids showed an overall seroprevalence for T. gondii of 63%. The risk factor study including data of 311 small exotic cats of 10 species resulted in a final generalized linear mixed model comprised of five variables: the likelihood of seropositivity increased statistically significantly with “Age”, while feeding “Cattle: frozen” relative to “Cattle: fresh”, “Outdoor housing fenced in on all sides”, “Mesh size 2–5 cm” relative to “Mesh size > 5 cm” and “Wearing gloves: yes” had statistically significant protective effects.
Conclusions
Wild felids, including endangered species, kept in human care in European and Middle Eastern institutions, are widely exposed to T. gondii. Risk factor analysis revealed that feeding previously frozen tissues, keeping animals in enclosures that are fenced on all sides using fences with small mesh sizes, and wearing gloves when working inside enclosures seem to be the most relevant protective measures to prevent T. gondii infections in these animals
Related collections
Most cited references87

- Record: found
- Abstract: found
- Article: found
Toxoplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact
- Record: found
- Abstract: found
- Article: not found
Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii
- Record: found
- Abstract: found
- Article: not found