- Record: found
- Abstract: found
- Article: found
Glutaminase-containing microvesicles from HIV-1-infected macrophages and immune-activated microglia induce neurotoxicity
Read this article at
Abstract
Background
HIV-1-infected and/or immune-activated microglia and macrophages are pivotal in the pathogenesis of HIV-1-associated neurocognitive disorders (HAND). Glutaminase, a metabolic enzyme that facilitates glutamate generation, is upregulated and may play a pathogenic role in HAND. Our previous studies have demonstrated that glutaminase is released to the extracellular fluid during HIV-1 infection and neuroinflammation. However, key molecular mechanisms that regulate glutaminase release remain unknown. Recent advances in understanding intercellular trafficking have identified microvesicles (MVs) as a novel means of shedding cellular contents. We posit that during HIV-1 infection and immune activation, microvesicles may mediate glutaminase release, generating excessive and neurotoxic levels of glutamate.
Results
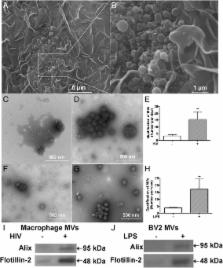
MVs isolated through differential centrifugation from cell-free supernatants of monocyte-derived macrophages (MDM) and BV2 microglia cell lines were first confirmed in electron microscopy and immunoblotting. As expected, we found elevated number of MVs, glutaminase immunoreactivities, as well as glutaminase enzyme activity in the supernatants of HIV-1 infected MDM and lipopolysaccharide (LPS)-activated microglia when compared with controls. The elevated glutaminase was blocked by GW4869, a neutral sphingomyelinase inhibitor known to inhibit MVs release, suggesting a critical role of MVs in mediating glutaminase release. More importantly, MVs from HIV-1-infected MDM and LPS-activated microglia induced significant neuronal injury in rat cortical neuron cultures. The MV neurotoxicity was blocked by a glutaminase inhibitor or GW4869, suggesting that the neurotoxic potential of HIV-1-infected MDM and LPS-activated microglia is dependent on the glutaminase-containing MVs.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: found
Extracellular vesicles as mediators of neuron-glia communication
- Record: found
- Abstract: found
- Article: not found
Regulation of glutaminase activity and glutamine metabolism.
- Record: found
- Abstract: found
- Article: not found