- Record: found
- Abstract: found
- Article: found
Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia
Read this article at
Abstract
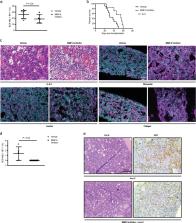
Specific and reciprocal interactions with the bone marrow microenvironment (BMM) govern the course of hematological malignancies. Matrix metalloproteinase-9 (MMP-9), secreted by leukemia cells, facilitates tumor progression via remodeling of the extracellular matrix (ECM) of the BMM. Hypothesizing that leukemias may instruct the BMM to degrade the ECM, we show, that MMP-9-deficiency in the BMM prolongs survival of mice with BCR-ABL1-induced B-cell acute lymphoblastic leukemia (B-ALL) compared with controls and reduces leukemia-initiating cells. MMP-9-deficiency in the BMM leads to reduced degradation of proteins of the ECM and reduced invasion of B-ALL. Using various in vivo and in vitro assays, as well as recipient mice deficient for the receptor for tumor necrosis factor (TNF) α (TNFR1) we demonstrate that B-ALL cells induce MMP-9-expression in mesenchymal stem cells (MSC) and possibly other cells of the BMM via a release of TNFα. MMP-9-expression in MSC is mediated by activation of nuclear factor kappa B (NF-κB) downstream of TNFR1. Consistently, knockdown of TNF-α in B-ALL-initiating cells or pharmacological inhibition of MMP-9 led to significant prolongation of survival in mice with B-ALL. In summary, leukemia cell-derived Tnfα induced MMP-9-expression by the BMM promoting B-ALL progression. Inhibition of MMP-9 may act as an adjunct to existing therapies.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis.
- Record: found
- Abstract: found
- Article: not found
MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis.
- Record: found
- Abstract: found
- Article: not found