- Record: found
- Abstract: found
- Article: found
Two New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
Read this article at
Abstract
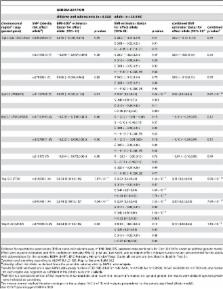
Meta-analyses of population-based genome-wide association studies (GWAS) in adults have recently led to the detection of new genetic loci for obesity. Here we aimed to discover additional obesity loci in extremely obese children and adolescents. We also investigated if these results generalize by estimating the effects of these obesity loci in adults and in population-based samples including both children and adults. We jointly analysed two GWAS of 2,258 individuals and followed-up the best, according to lowest p-values, 44 single nucleotide polymorphisms (SNP) from 21 genomic regions in 3,141 individuals. After this DISCOVERY step, we explored if the findings derived from the extremely obese children and adolescents (10 SNPs from 5 genomic regions) generalized to (i) the population level and (ii) to adults by genotyping another 31,182 individuals (GENERALIZATION step). Apart from previously identified FTO, MC4R, and TMEM18, we detected two new loci for obesity: one in SDCCAG8 (serologically defined colon cancer antigen 8 gene; p = 1.85×10 −8 in the DISCOVERY step) and one between TNKS (tankyrase, TRF1-interacting ankyrin-related ADP-ribose polymerase gene) and MSRA (methionine sulfoxide reductase A gene; p = 4.84×10 −7), the latter finding being limited to children and adolescents as demonstrated in the GENERALIZATION step. The odds ratios for early-onset obesity were estimated at ∼1.10 per risk allele for both loci. Interestingly, the TNKS/MSRA locus has recently been found to be associated with adult waist circumference. In summary, we have completed a meta-analysis of two GWAS which both focus on extremely obese children and adolescents and replicated our findings in a large followed-up data set. We observed that genetic variants in or near FTO, MC4R, TMEM18, SDCCAG8, and TNKS/ MSRA were robustly associated with early-onset obesity. We conclude that the currently known major common variants related to obesity overlap to a substantial degree between children and adults.
Author Summary
Genome-wide association studies (GWAS) have successfully contributed to the detection of genetic variants involved in body-weight regulation. We jointly analysed two GWAS for early-onset extreme obesity in 2,258 individuals of European origin and followed-up the findings in 3,141 individuals. Evidence for association of markers in two new genetic loci was shown ( SDCCAG8 on chromosome 1q43–q44 and between TNKS/MSRA on chromosome 8p23.1). We also re-identified variants in or near FTO, MC4R, and TMEM18 to be associated with extreme obesity. In addition, we assessed the effect of the markers in 31,182 obese, lean, normal weight, and unselected individuals from population-based samples and showed that the variants near FTO, MC4R, TMEM18, and SDCCAG8 were consistently associated with obesity. For variants of TNKS/MSRA, the obesity association was limited to children and adolescents. In summary, we detected two new obesity loci and confirmed that the currently known major common variants related to obesity overlap to a substantial degree between children and adults.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Six new loci associated with body mass index highlight a neuronal influence on body weight regulation.
- Record: found
- Abstract: found
- Article: not found
The human obesity gene map: the 2005 update.
- Record: found
- Abstract: found
- Article: not found