- Record: found
- Abstract: found
- Article: found
TRIM28 facilitates type I interferon activation by targeting TBK1
Read this article at
Abstract
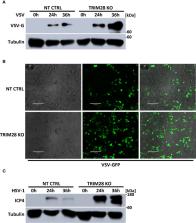
Type I interferons play a fundamental role in innate host defense against viral infections by eliciting the induction of an antiviral gene program that serves to inhibit viral replication. Activation of type I interferon is regulated by the IRF3 transcription factor, which undergoes phosphorylation-dependent activation by the upstream kinase, TBK1, during viral infection. However, the mechanisms by which TBK1 achieves activation to support signaling to IRF3 remain incompletely understood. Here we identified the E3 ubiquitin ligase, tripartite motif containing 28 (TRIM28), as a positive regulator of type I interferon activation by facilitating TBK1 signaling. Genetic deletion of TRIM28 via CRISPR-Cas9 editing resulted in impaired type I interferon activation upon both RNA and DNA virus challenge, corresponding with increased susceptibility to virus infections in TRIM28 knockout cells. Mechanistically, TRIM28 interacted with TBK1 and mediated the assembly of K63-linked ubiquitin chains onto TBK1, a post-translational modification shown to augment TBK1 signal transmission events. TRIM28 knockout cells further displayed defective TBK1 phosphorylation and complex assembly with IRF3, resulting in impaired IRF3 phosphorylation. Altogether, our data demonstrate TBK1 to be a novel substrate for TRIM28 and identify TRIM28 as an essential regulatory factor in controlling innate antiviral immune responses.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity.
- Record: found
- Abstract: found
- Article: not found
KAP1 controls endogenous retroviruses in embryonic stem cells.
- Record: found
- Abstract: not found
- Article: not found