- Record: found
- Abstract: found
- Article: found
Seasonality and transmissibility of Plasmodium ovale in Bagamoyo District, Tanzania
Read this article at
Abstract
Background
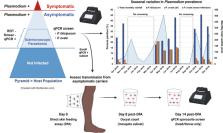
Plasmodium ovale is a neglected malarial parasite that can form latent hypnozoites in the human liver. Over the last decade, molecular surveillance studies of non-falciparum malaria in Africa have highlighted that P. ovale is circulating below the radar, including areas where Plasmodium falciparum is in decline. To eliminate malaria where P. ovale is endemic, a better understanding of its epidemiology, asymptomatic carriage, and transmission biology is needed.
Methods
We performed a pilot study on P. ovale transmission as part of an ongoing study of human-to-mosquito transmission of P. falciparum from asymptomatic carriers. To characterize the malaria asymptomatic reservoir, cross-sectional qPCR surveys were conducted in Bagamoyo, Tanzania, over three transmission seasons. Positive individuals were enrolled in transmission studies of P. falciparum using direct skin feeding assays (DFAs) with Anopheles gambiae s.s. (IFAKARA strain) mosquitoes. For a subset of participants who screened positive for P. ovale on the day of DFA, we incubated blood-fed mosquitoes for 14 days to assess sporozoite development.
Results
Molecular surveillance of asymptomatic individuals revealed a P. ovale prevalence of 11% (300/2718), compared to 29% (780/2718) for P. falciparum. Prevalence for P. ovale was highest at the beginning of the long rainy season (15.5%, 128/826) in contrast to P. falciparum, which peaked later in both the long and short rainy seasons. Considering that these early-season P. ovale infections were low-density mono-infections (127/128), we speculate many were due to hypnozoite-induced relapse. Six of eight P. ovale-infected asymptomatic individuals who underwent DFAs successfully transmitted P. ovale parasites to A. gambiae.
Conclusions
Plasmodium ovale is circulating at 4–15% prevalence among asymptomatic individuals in coastal Tanzania, largely invisible to field diagnostics. A different seasonal peak from co-endemic P. falciparum, the capacity to relapse, and efficient transmission to Anopheles vectors likely contribute to its persistence amid control efforts focused on P. falciparum.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally.
- Record: found
- Abstract: found
- Article: found
Plasmodium malariae and P. ovale genomes provide insights into malaria parasite evolution

- Record: found
- Abstract: found
- Article: found
