- Record: found
- Abstract: found
- Article: found
Extreme inbreeding in a European ancestry sample from the contemporary UK population

Read this article at
Abstract
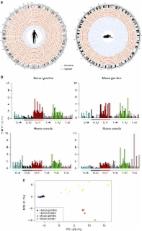
In most human societies, there are taboos and laws banning mating between first- and second-degree relatives, but actual prevalence and effects on health and fitness are poorly quantified. Here, we leverage a large observational study of ~450,000 participants of European ancestry from the UK Biobank (UKB) to quantify extreme inbreeding (EI) and its consequences. We use genotyped SNPs to detect large runs of homozygosity (ROH) and call EI when >10% of an individual’s genome comprise ROHs. We estimate a prevalence of EI of ~0.03%, i.e., ~1/3652. EI cases have phenotypic means between 0.3 and 0.7 standard deviation below the population mean for 7 traits, including stature and cognitive ability, consistent with inbreeding depression estimated from individuals with low levels of inbreeding. Our study provides DNA-based quantification of the prevalence of EI in a European ancestry sample from the UK and measures its effects on health and fitness traits.
Abstract
Mating between first or second-degree relatives is prohibited in most countries, yet it occurs and is under-studied. Here, Yengo et al. use large runs of homozygosity from the UK Biobank resource to provide DNA-based quantification of extreme inbreeding and its consequence for health and other complex traits.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
The genetics of inbreeding depression.
- Record: found
- Abstract: found
- Article: not found
The genetic basis of inbreeding depression.
- Record: found
- Abstract: found
- Article: found