- Record: found
- Abstract: found
- Article: found
Activating transcription factor 4 mediates adaptation of human glioblastoma cells to hypoxia and temozolomide
Read this article at
Abstract
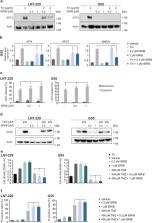
The integrated stress response (ISR) is a central cellular adaptive program that is activated by diverse stressors including ER stress, hypoxia and nutrient deprivation to orchestrate responses via activating transcription factor 4 (ATF4). We hypothesized that ATF4 is essential for the adaptation of human glioblastoma (GB) cells to the conditions of the tumor microenvironment and is contributing to therapy resistance against chemotherapy. ATF4 induction in GB cells was modulated pharmacologically and genetically and investigated in the context of temozolomide treatment as well as glucose and oxygen deprivation. The relevance of the ISR was analyzed by cell death and metabolic measurements under conditions to approximate aspects of the GB microenvironment. ATF4 protein levels were induced by temozolomide treatment. In line, ATF4 gene suppressed GB cells (ATF4sh) displayed increased cell death and decreased survival after temozolomide treatment. Similar results were observed after treatment with the ISR inhibitor ISRIB. ATF4sh and ISRIB treated GB cells were sensitized to hypoxia-induced cell death. Our experimental study provides evidence for an important role of ATF4 for the adaptation of human GB cells to conditions of the tumor microenvironment characterized by low oxygen and nutrient availability and for the development of temozolomide resistance. Inhibiting the ISR in GB cells could therefore be a promising therapeutic approach.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma
- Record: found
- Abstract: found
- Article: not found