- Record: found
- Abstract: found
- Article: found
Macrophages Are Key Regulators of Stem Cells during Skeletal Muscle Regeneration and Diseases
Read this article at
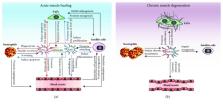
Abstract
Muscle regeneration is a closely regulated process that involves a variety of cell types such as satellite cells, myofibers, fibroadipogenic progenitors, endothelial cells, and inflammatory cells. Among these different cell types, macrophages emerged as a central actor coordinating the different cellular interactions and biological processes. Particularly, the transition of macrophages from their proinflammatory to their anti-inflammatory phenotype was shown to regulate inflammation, myogenesis, fibrosis, vascularization, and return to homeostasis. On the other hand, deregulation of macrophage accumulation or polarization in chronic degenerative muscle disorders was shown to impair muscle regeneration. Considering the key roles of macrophages in skeletal muscle, they represent an attractive target for new therapeutic approaches aiming at mitigating various muscle disorders. This review aims at summarizing the novel insights into macrophage heterogeneity, plasticity, and functions in skeletal muscle homeostasis, regeneration, and disease.
Related collections
Most cited references136
- Record: found
- Abstract: found
- Article: not found
Regulatory interactions between muscle and the immune system during muscle regeneration.
- Record: found
- Abstract: found
- Article: not found
Inflammatory processes in muscle injury and repair.
- Record: found
- Abstract: found
- Article: not found