- Record: found
- Abstract: found
- Article: found
From embryos to embryoids: How external signals and self-organization drive embryonic development
Read this article at
Summary
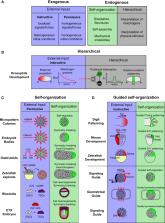
Embryonic development has been traditionally seen as an inductive process directed by exogenous maternal inputs and extra-embryonic signals. Increasing evidence, however, is showing that, in addition to exogenous signals, the development of the embryo involves endogenous self-organization. Recently, this self-organizing potential has been highlighted by a number of stem cell models known as embryoids that can recapitulate different aspects of embryogenesis in vitro. Here, we review the self-organizing behaviors observed in different embryoid models and seek to reconcile this new evidence with classical knowledge of developmental biology. This analysis leads to reexamine embryonic development as a guided self-organizing process, where patterning and morphogenesis are controlled by a combination of exogenous signals and endogenous self-organization. Finally, we discuss the multidisciplinary approach required to investigate the genetic and cellular basis of self-organization.
Graphical abstract
Abstract
This perspective analyzes the different self-organizing processes observed in embryoid models, putting emphasis on symmetry breaking and self-assembly. Within this analysis, embryonic development is re-interpreted as a guided self-organizing process where epiblast self-organization is modulated by extra-embryonic signals. Finally, the authors present a multidisciplinary approach to study the genetic and cellular basis of self-organization.
Related collections
Most cited references79
- Record: found
- Abstract: not found
- Article: not found
The Chemical Basis of Morphogenesis
- Record: found
- Abstract: found
- Article: not found
Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development.
- Record: found
- Abstract: found
- Article: not found
