- Record: found
- Abstract: found
- Article: found
PLCβ-Mediated Depletion of PIP 2 and ATP-Sensitive K + Channels Are Involved in Arginine Vasopressin-Induced Facilitation of Neuronal Excitability and LTP in the Dentate Gyrus
Read this article at
Abstract
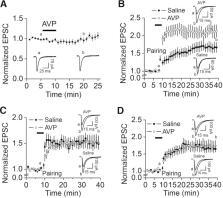
Arginine vasopressin (AVP) serves as a neuromodulator in the brain. The hippocampus is one of the major targets for AVP, as it has been demonstrated that the hippocampus receives vasopressinergic innervation and expresses AVP receptors. The dentate gyrus (DG) granule cells (GCs) serve as a gate governing the inflow of information to the hippocampus. High densities of AVP receptors are expressed in the DG GCs. However, the roles and the underlying cellular and molecular mechanisms of AVP in the DG GCs have not been determined. We addressed this question by recording from the DG GCs in rat hippocampal slices. Our results showed that application of AVP concentration-dependently evoked an inward holding current recorded from the DG GCs. AVP depolarized the DG GCs and increased their action potential firing frequency. The excitatory effects of AVP were mediated by activation of V 1a receptors and required the function of phospholipase Cβ (PLCβ). Whereas intracellular Ca 2+ release and protein kinase C activity were unnecessary, PLCβ-induced depletion of phosphatidylinositol 4,5-bisphosphate was involved in AVP-evoked excitation of the DG GCs. AVP excited the DG GCs by depression of the ATP-sensitive K + channels, which were required for AVP-elicited facilitation of long-term potentiation at the perforant path–GC synapses. Our results may provide a cellular and molecular mechanism to explain the physiological functions of AVP, such as learning and memory, and pathologic disorders like anxiety.
Related collections
Most cited references98
- Record: found
- Abstract: found
- Article: not found
Are the dorsal and ventral hippocampus functionally distinct structures?
- Record: found
- Abstract: found
- Article: not found
Functional organization of the hippocampal longitudinal axis.
- Record: found
- Abstract: found
- Article: not found