- Record: found
- Abstract: found
- Article: not found
CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2

Read this article at
Abstract
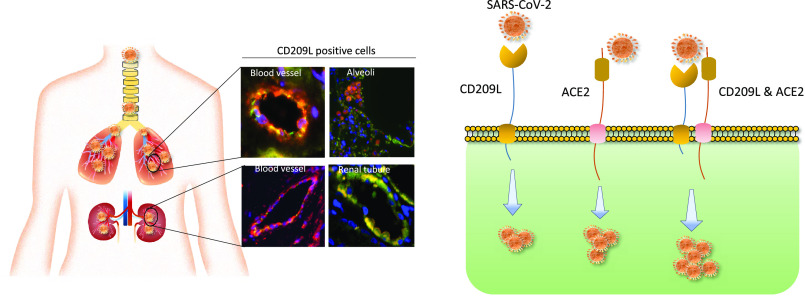
As the COVID-19 pandemic continues to spread, investigating the processes underlying the interactions between SARS-CoV-2 and its hosts is of high importance. Here, we report the identification of CD209L/L-SIGN and the related protein CD209/DC-SIGN as receptors capable of mediating SARS-CoV-2 entry into human cells. Immunofluorescence staining of human tissues revealed prominent expression of CD209L in the lung and kidney epithelia and endothelia. Multiple biochemical assays using a purified recombinant SARS-CoV-2 spike receptor-binding domain (S-RBD) or S1 encompassing both N termal domain and RBD and ectopically expressed CD209L and CD209 revealed that CD209L and CD209 interact with S-RBD. CD209L contains two N-glycosylation sequons, at sites N92 and N361, but we determined that only site N92 is occupied. Removal of the N-glycosylation at this site enhances the binding of S-RBD with CD209L. CD209L also interacts with ACE2, suggesting a role for heterodimerization of CD209L and ACE2 in SARS-CoV-2 entry and infection in cell types where both are present. Furthermore, we demonstrate that human endothelial cells are permissive to SARS-CoV-2 infection, and interference with CD209L activity by a knockdown strategy or with soluble CD209L inhibits virus entry. Our observations demonstrate that CD209L and CD209 serve as alternative receptors for SARS-CoV-2 in disease-relevant cell types, including the vascular system. This property is particularly important in tissues where ACE2 has low expression or is absent and may have implications for antiviral drug development.
Abstract
In human endothelial cells, CD209L acts as a receptor for SARS-CoV-2; together with ACE2, it can function as a co-receptor. Blocking CD209L activity inhibited virus entry, indicating a novel target for development of antiviral drugs.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study

- Record: found
- Abstract: found
- Article: found
