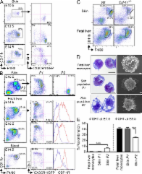
Epidermal Langerhans cells (LCs) belong to the DC family; a small group of tissue hematopoietic cells that specialize in the induction of adaptive immune responses. Similar to most DCs, LCs are well equipped to capture, process, and present peptide-bound MHC complexes on the cell surface and migrate from the epidermis to the skin-draining lymph nodes to present cutaneous antigens to T lymphocytes (Merad et al., 2008; Romani et al., 2010). LCs exhibit specific differentiation and homeostatic features, which distinguish them from other DC populations. For example, whereas DC development and homeostasis are critically controlled by fms-like tyrosine kinase 3 (Flt3) ligand (Flt3L) and its receptor Flt3 (Merad and Manz, 2009), mice lacking Flt3 or Flt3L have normal numbers of LCs in vivo (Ginhoux et al., 2009; Kingston et al., 2009). In contrast, the receptor for colony stimulating factor-1 (CSF-1R) is required for LCs to develop (Ginhoux et al., 2006), but is dispensable for the development of lymphoid tissue resident DCs (Ginhoux et al., 2009; Witmer-Pack et al., 1993). In contrast to other DCs, which are constantly replaced by a circulating pool of BM-derived committed precursors, LCs maintain themselves in situ throughout life, independent of any input from the BM (Merad et al., 2002). Furthermore, LCs resist high-dose ionized radiation and remain of host origin after lethal irradiation and reconstitution with donor congenic BM (Merad et al., 2002). The origin of the precursors that give rise to tissue DCs are beginning to be characterized (Geissmann et al., 2010). For example, the macrophage and DC precursor gives rise to monocytes and to the common DC precursor, which has lost monocyte/macrophage differential potential and gives rise exclusively to DCs. However, none of these progenitors contribute to LC homeostasis in adult mice. In contrast, adult LC homeostasis is maintained by a pool of LC precursors that take residence in the skin before birth (Romani et al., 1986; Chang-Rodriguez et al., 2005; Chorro et al., 2009). However, the origin and the developmental regulation of these embryonic LC precursors remain unknown. Two major hematopoietic sites contribute to blood cell formation during embryogenesis (Tavian and Péault, 2005; Orkin and Zon, 2008). In mice, the first hematopoietic progenitor appears in the extra-embryonic yolk sac (YS) shortly after the onset of gastrulation, around embryonic age (E) 7.0, leading to the initiation of primitive hematopoiesis, which consists mainly of erythrocytes and macrophages (Moore and Metcalf, 1970; Bertrand et al., 2005). Primitive macrophages spread into the embryo with the onset of blood circulation around E9.0 (Lichanska and Hume, 2000). After E8.5, with the determination of the intraembryonic mesoderm toward the hematopoietic lineage, a new wave of hematopoietic progenitors is generated within the embryo proper, first in the paraaortic splanchnopleura region, and then in the aorta, gonads, and mesonephros (AGM) region (Medvinsky et al., 1993; Godin et al., 1993). The hematopoietic stem cells generated within the AGM will lead to the establishment of definitive hematopoiesis (Orkin and Zon, 2008). Around E10.5, YS- and AGM-derived hematopoietic progenitors colonize the fetal liver (Kumaravelu et al., 2002), which serves as a major hematopoietic organ after E11.5, generating all hematopoietic lineages, including monocytes (Naito et al., 1990). We recently showed that microglia, the resident macrophage population of the central nervous system, arise exclusively from YS-derived primitive myeloid progenitors that appear before E8.0 (Ginhoux et al., 2010). Interestingly, similar to LCs, microglial cells are also dependent on the CSF-1R for their development (Ginhoux et al., 2010), and they resist high-dose ionized radiation and maintain themselves in situ, independent of any input from BM precursors (Ajami et al., 2007; Mildner et al., 2007). These shared cytokine requirements and homeostatic properties may suggest a common developmental origin of these two cell types. In this study, we examined the contribution of embryonic myeloid precursors to LC homeostasis in adult mice. Using in vivo lineage-tracing studies and in utero adoptive transfer strategies, we show that adult LCs derive exclusively from embryonic precursors of both YS-derived primitive macrophage and fetal liver–derived monocyte origin. RESULTS LC precursors are recruited to the skin before birth Adult LCs derive from hematopoietic precursors that take residence in the skin before birth, and before the onset of BM hematopoiesis. Early studies revealed that myeloid-like cells expressing the myeloid markers F4/80 and CD11b were present in the skin during the later stages of embryonic development (Ginhoux and Merad, 2010). These cells were considered LC precursors because they were proliferating actively (Chang-Rodriguez et al., 2005; Chorro et al., 2009) and lacked mature LC markers such as MHC class II and langerin, which were acquired after birth (Romani et al., 1986; Tripp et al., 2004). However, these studies were mainly correlative, and the potential contribution of postnatal hematopoietic progenitors to the adult LC pool in the steady state was never formally addressed. To exclude the possibility that a wave of perinatal circulating hematopoietic precursors, such as monocytes, contributes to adult LC homeostasis, we reconstituted C57BL/6 CD45.2+ newborns that were sublethally irradiated in the first 24 h after birth with adult BM hematopoietic cells isolated from CD45.1+ congenic mice. Donor CD45.1+ cell engraftment was measured in the blood 3 mo after transplantation and showed that >30% of blood circulating leukocytes, including B and T lymphocytes, monocytes, and macrophages from lymphoid and nonlymphoid tissues, were of donor origin (Fig. 1, A and B). In contrast, >95% of LCs were of host origin at this time point (Fig. 1, A and B). Similar results were obtained after reconstitution with E14.5 fetal liver cells (unpublished data). LC chimerism was also monitored at later time points, and LCs remained of host origin for >7 mo after reconstitution (Fig. 1 B). Similar results were obtained when microglia were analyzed (Fig. 1 B), as previously reported (Ginhoux et al., 2010). Thus, similar to microglia, these results suggest that LCs are maintained independent of circulating monocytes and instead rely on local radioresistant precursors that colonize the epidermis before birth. Figure 1. Adult LCs arise from embryonic precursors. Newborn CD45.2+ mice were reconstituted with bone marrow cells isolated from adult CD45.1+ mice. (A) Percentage donor-derived cell populations 3 mo after newborn transplantation (TX). Each data point represents a single mouse. Bars represent means of data from 4 pooled experiments (n = 11). The gating strategy for each leukocyte population was described previously (Ginhoux et al., 2010). (B) Percentage donor-derived monocytes, microglial cells, and LCs at different time points after reconstitution. Bars represent means of data from 4 pooled experiments (n = 4–11). ***, P 30% eYFP+ cells in embryos treated with 4’OHT at E8.5 (Fig. 7 E). In contrast, the proportion of eYFP+ cells among population P2 was similar to that of adult LCs and reached 6% eYFP+ cells in embryos treated with 4’OHT at E7.5 and 30% eYFP+ cells in embryos treated with 4’OHT at E8.5 (Fig. 7 E). Based on these results, we hypothesized that fetal liver–derived monocytes are recruited to the prospective dermis from E13.5 to E16.5 (population P1), where they differentiate into LC precursors (population P2) in a CSF-1R–dependent manner, before their recruitment to the epidermis around E16.5. To definitely determine whether fetal liver monocytes can give rise to LC precursors in the fetal skin, we adoptively transferred fetal liver monocytes isolated from E13.5–E14.5 congenic C57BL/6 Cx3cr1gfp/+ CD45.1+ embryos into E13.5–E14.5 C57BL/6 CD45.2+ host embryos in utero (2–3.105 monocytes per embryo; Fig. 8 A). Donor-derived monocytes were detected in the blood (Fig. 8, B and D) and in the skin (Fig. 8, C and D) of recipient embryos as early as 3 h after adoptive transfer, but were always absent from the brain (not depicted). Engraftment variability observed in recipient mice was embryo dependent and not experiment dependent (unpublished data). Analysis at later time points after transfer (48 and 72 h corresponding to E16.5 and E17.5, respectively) revealed that adoptively transferred fetal liver monocytes (population P1) give rise to transitional cells, that down-regulated Gr-1 and up-regulated CX3CR1 before differentiating into LC precursors (population P2; Fig. 8, E and F). By E19.5 (5 d after transfer), all donor monocytes had differentiated into P2-like cells (Fig. 8, E [bottom] and F). Noninjected embryos or embryos injected with Ter119+ erythroid progenitors were used as negative controls (Fig. 8, D and G). Figure 8. Fetal liver monocytes differentiate into LC precursors. Monocytes were purified from E13.5–E14.5 fetal liver of Cx3cr1gfp/+ CD45.1+ mice and adoptively transferred in utero into unconditioned E13.5–E14.5 CD45.2+ congenic embryos. (A–C) Flow cytometry analysis of the progeny of adoptively transferred CD45.1+ monocytes (A) in the blood (B) and skin (C) 3 h after injection. (A) Gating strategy (top) for monocytes among fetal liver leukocytes (DAPI−CD45+), purity after sorting (middle), and profile of expression for Gr-1 and CX3CR1/GFP (bottom). (B and C) Percentage CD45.1+ donor-derived monocytes among total CD45+ cells (top), expression of CD11b and F4/80 (middle), and Gr-1 and CX3CR1/GFP (bottom) among indicated populations. (D) Percentage cells derived from donor monocytes for each injected embryo (n = 7) in E14.5 blood and skin 3 h after transfer. Control represents noninjected embryos (n = 9). Bars show means of data from one representative experiment of three. (E) Percentage populations P1 (CD11bhiF480lo) and P2 (CD11bloF480hi) on gated DAPI−CD11b+F480+CD45.1+ donor-derived cells isolated from the skin at indicated time points after transfer (E14.5) and their corresponding profile of expression for Gr-1 and CX3CR1/GFP. (F) Percentage population P2 on gated DAPI−CD11b+F480+CD45.1+ donor derived cells isolated from the skin at indicated time points after transfer. Error bars represent mean ± SEM of pooled data from two experiments (n = 3 to 8). (G) Graph shows the percentage of skin CD11b+F4/80+ cells derived from donor fetal liver monocytes (white squares) or donor fetal liver Ter119+ erythroid progenitors (gray squares; n = 4–11) for each injected embryo at the indicated time points. Bars represent means of data at each time point. ***, P 98% purity after post-sort verification, and were adoptively transferred in utero into the peritoneal cavity of time-mated E13.5–E14.5 C57BL/6 CD45.2+ host embryos (2–3 × 105 cells per embryo) as previously described (Chan et al., 2007). In brief, a full-depth midline laparotomy was performed to expose the gravid uterus. Identification of the fetal abdomen through the translucent uterine wall allowed delivery of the monocytes by the intraperitoneal route. Cells were injected in 10 µl of saline using a 33-gauge needle, and the mice were allowed to recover in a warmed cage after closure of the abdominal wound with 6/0 silk sutures. Cell suspension preparations. Skin cell suspensions were isolated as previously described (Ginhoux et al., 2007) and analyzed by flow cytometry. In brief, mouse ears (split in dorsal and ventral parts) or whole skin (starting from E16.5) were first incubated for 60 min in HBSS containing Dispase (2.4 mg/ml, working activity of 1.7 U/mg; Invitrogen) to allow for separation of dermal and epidermal sheets before subsequent Collagenase incubation. All tissues from adult mice, newborns, or embryos were cut into small pieces, incubated in HBSS containing 10% fetal bovine serum, and Collagenase type IV (0.2 mg/ml, working activity of 770 U/mg; Sigma-Aldrich; 2 h for adult tissues and 1 h for newborns and embryonic tissues), and then syringed through a 19-gauge needle to obtain a homogeneous cell suspension. Embryonic blood cells were collected after decapitation in PBS 10 mM EDTA and red blood cells were lysed. Analysis was performed by flow cytometry, gating on singlets of DAPI− CD45+ cells. Flow cytometry and cell cycle analysis. Flow cytometric studies were performed using a BD FACSCanto and a BD LSR II (BD) with subsequent data analysis using FlowJo software (Tree Star). Fluorochrome- or biotin-conjugated mAbs specific for mouse B220 (clone RA3-6B2), MHC class II I-A/I-E (clone M5/114.15.2), CD11b (clone M1/70), CD45 (clone 30F11), CD45.1 (clone A20), CD45.2 (clone 104), CSF-1R (clone AFS98), Gr-1Ly6C/G (clone RB6-8C5), and CD3 (clone 17A2), the corresponding isotype controls and the secondary reagents (allophycocyanin, peridinin chlorophyll protein, and phycoerythrin–indotricarbocyanine–conjugated streptavidin) were purchased either from BD or eBioscience. Anti-F4/80 (A3-1) mAb was purchased from Serotec. Polyclonal antibody to langerin (E17) was purchased from Santa Cruz Biotechnology, Inc. Intracellular staining against langerin was performed with the BD Cytofix/Cytoperm kit (BD) according to the manufacturer’s protocol. For cell cycle analysis, stained cell suspensions were first fixed in 2% paraformaldehyde in PBS solution (30 min), and then washed and fixed in 70% ethanol (2 h). After washing, cells were incubated overnight with 10 mM DAPI in PBS solution to stain cell DNA content before data acquisition. Immunohistochemistry analysis. Time-mated E12.5 and E16.5 Cx3cr1gfp/+ embryos were fixed in 2% paraformaldehyde solution containing 30% sucrose overnight and snap-frozen in OCT. 20-µm frozen sections were labeled with biotinylated anti-F4/80 (Serotec) followed by Dy649-conjugated streptavidin (Jacksons ImmunoResearch Laboratories). Sections were counterstained with DAPI for nuclei staining and analyzed with a confocal microscope (FV-1000 confocal system; Olympus). For whole-mount X-Gal staining, embryos were dissected and fixed immediately in a solution containing 0.2% glutaraldehyde for 1 h on ice, washed three times at room temperature in a buffer containing 0.1% sodium deoxycholate and 0.02% NP-40, and stained with X-Gal at 37°C overnight. Imaging procedures. For cytospin and SEM preparations, corresponding myeloid progenitors were sorted using a FACSAria II (BD) to achieve 98% purity. For cytospin, purified cells were spun onto glass slides, dried overnight, stained using the Hema 3 System (Thermo Fisher Scientific), and rinsed in distilled water. Images were analyzed using an Eclipse E800 microscope (Nikon) at a 10 × 60-fold magnification. For SEM imaging, sorted cells were coated on a poly-lysine (Sigma-Aldrich) pretreated glass coverslip for 15 min at room temperature, fixed in 2.5% glutaraldehyde 0.1 M phosphate buffer for 1 h, pH 7.4, at room temperature and washed 2 times in PBS. After post-fixation with 1% osmium tetroxide (Ted Pella Inc.) at room temperature for 1 h, cells were washed in deionized water, dehydrated with a graded series of ethanol immersions starting at 25–100%, and critical point dried (CPD 030; Bal-Tec). The glass coverslip was then laid on an adhesive film on an SEM sample holder and firmly touched with an adhesive sample holder. The surface on which the cells were deposited and the adhesive surfaces were coated with 5 nm of platinum by sputter coating in a high-vacuum sputtering device (SCD005 sputter coater; Bal-Tec). The coated samples were examined with a field emission scanning electron microscope (JSM-6701F; JEOL) at an acceleration voltage of 8 kV using the in-lens secondary electron detector, with a working distance ranging from 7.5 to 8.3 mm. Magnification ×10,000, except for subepidermal mesenchyme LC precursors, which had a magnification of ×7,000 (Fig. 7). For multiphoton imaging of embryos, pregnant mice were euthanized by CO2 asphyxiation, the Cx3cr1gfp/+ or Csf1rgfp/+ embryos were isolated and mounted on a customized Petri dish for multiphoton imaging on a LaVision Biotec TrimScope equipped with a 20× water immersion objective. Evans blue was injected intravenously to label blood vessels. The whole intact embryo was incubated in DAPI for 5 min to label the surface of the skin to distinguish the exact localization of the cells in relation to the surface. For imaging, the explanted embryo was exposed to polarized laser light at a wavelength of 950 nm. Three-dimensional (x,y,z) image stacks of the skin in the vicinity of the upper limb were acquired (1-µm spacing in z-axis over a total distance of up to 130–160 µm). For static three-dimensional images of the embryonic skin at E12.5, embryos were positioned upright in Agarose gels. Acquired image stacks were processed using Imaris software (Bitplane). Statistical analysis. For statistical analysis, repeated measures of ANOVA and Mann-Whitney tests (with a 95% confidence) were performed using Prism 4.0 (GraphPad Software). All p-values are two-tailed.

 Tissue-resident macrophages are mostly derived from embryonic progenitors. Scott
et al. develop a mouse model to specifically deplete Kupffer cells (KC)
in vivo and show that monocyte-derived cells can repopulate KC niche and behave similar to
their embryonically-derived counterparts.
Tissue-resident macrophages are mostly derived from embryonic progenitors. Scott
et al. develop a mouse model to specifically deplete Kupffer cells (KC)
in vivo and show that monocyte-derived cells can repopulate KC niche and behave similar to
their embryonically-derived counterparts.