- Record: found
- Abstract: found
- Article: found
Cotargeting EBV lytic as well as latent cycle antigens increases T-cell potency against lymphoma
Read this article at
Key Points
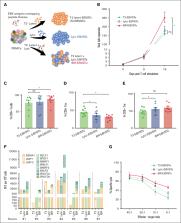
Visual Abstract
Abstract
The remarkable efficacy of Epstein-Barr virus (EBV)-specific T cells for the treatment of posttransplant lymphomas has not been reproduced for EBV-positive (EBV +) malignancies outside the transplant setting. This is because of, in part, the heterogeneous expression and poor immunogenicity of the viral antigens expressed, namely latent membrane proteins 1 and 2, EBV nuclear antigen 1, and BamHI A rightward reading frame 1 (type-2 [T2] latency). However, EBV lytic cycle proteins are also expressed in certain EBV + malignancies and, because several EBV lytic cycle proteins are abundantly expressed, have oncogenic activity, and likely contribute to malignancy, we sought and identified viral lytic-cycle transcripts in EBV + Hodgkin lymphoma biopsies. This provided the rationale for broadening the target antigen–specific repertoire of EBV-specific T cells (EBVSTs) for therapy. We stimulated, peripheral blood mononuclear cells from healthy donors and patients with EBV+ lymphoma with both lytic and latent cycle proteins to produce broad repertoire (BR) EBVSTs. Compared with T2 antigen-specific EBVSTs, BR-EBVSTs more rapidly cleared autologous EBV + tumors in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice and produced higher levels of proinflammatory cytokines that should reactivate the immunosuppressive tumor microenvironment leading to epitope spreading. Our results confirm that lytic cycle antigens are clinically relevant targets for EBV + lymphoma and underpin the rationale for integrating BR-EBVSTs as a therapeutic approach for relapsed/refractory EBV + lymphoma ( www.clinicaltrials.gov identifiers: #NCT01555892 and #NCT04664179), as well as for other EBV-associated malignancies.
Related collections
Most cited references68
- Record: found
- Abstract: not found
- Article: not found
Chimeric Antigen Receptor Therapy.

- Record: found
- Abstract: found
- Article: found
Cytokines in clinical cancer immunotherapy
- Record: found
- Abstract: found
- Article: not found
