- Record: found
- Abstract: found
- Article: found
Oral drugs in the treatment of metastatic colorectal cancer
Read this article at
Abstract
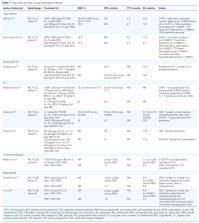
Colorectal cancer (CRC) is one of the most common forms of cancer, with an estimated 1.36 million new cases and almost 700,000 deaths annually. Approximately 21% of patients with CRC have metastatic disease at diagnosis. The objective of this article is to review the literature on the efficacy and safety of oral drugs available for the treatment of metastatic colorectal cancer (mCRC). Several such drugs have been developed, and fluoropyrimidines are the backbone of chemotherapy in this indication. They exert their antitumour activity by disrupting the synthesis and function of DNA and RNA. Oral fluoropyrimidines include prodrugs capecitabine, tegafur, eniluracil/5-fluorouracil, tegafur/uracil, tegafur/gimeracil/oteracil and trifluridine/tipiracil (FTD/TPI). Oral drugs offer several advantages over injectable formulations, including convenience, flexibility, avoidance of injection-related adverse events (AEs) and, in some circumstances, lower costs. However, oral drugs may not be suitable for patients with gastrointestinal obstruction or malabsorption, they may result in reduced treatment adherence and should not be co-administered with drugs that interfere with absorption or hepatic metabolism. Oral fluoropyrimidines such as capecitabine, as monotherapy or in combination with oxaliplatin, irinotecan or bevacizumab, are as effective as intravenous 5-fluorouracil (5-FU) in first-line treatment of mCRC. Other oral fluoropyrimidines, such as FTD/TPI, are effective in patients with mCRC who are refractory, intolerant or ineligible for 5-FU. In addition, oral fluoropyrimidines are used in adjuvant treatment of mCRC. Regorafenib is an oral multikinase inhibitor used in patients in whom several previous lines of therapy have failed. Frequent AEs associated with oral drugs used in the treatment of CRC include hand-foot syndrome and gastrointestinal and haematological toxicities.
Related collections
Most cited references96
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
ESMO consensus guidelines for the management of patients with metastatic colorectal cancer.
- Record: found
- Abstract: found
- Article: not found