- Record: found
- Abstract: found
- Article: found
From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators
Read this article at
Abstract
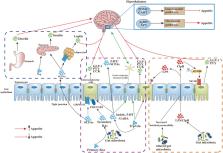
Feelings of hunger and satiety are the key determinants for maintaining the life of humans and animals. Disturbed appetite control may disrupt the metabolic health of the host and cause various metabolic disorders. A variety of factors have been implicated in appetite control, including gut microbiota, which develop the intricate interactions to manipulate the metabolic requirements and hedonic feelings. Gut microbial metabolites and components act as appetite-related signaling molecules to regulate appetite-related hormone secretion and the immune system, or act directly on hypothalamic neurons. Herein, we summarize the effects of gut microbiota on host appetite and consider the potential molecular mechanisms. Furthermore, we propose that the manipulation of gut microbiota represents a clinical therapeutic potential for lessening the development and consequence of appetite-related disorders.
Related collections
Most cited references234
- Record: found
- Abstract: found
- Article: not found
Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity.
- Record: found
- Abstract: found
- Article: not found