- Record: found
- Abstract: found
- Article: found
Spatial transcriptomics and neurofilament light chain reveal changes in lesion patterns in murine autoimmune neuroinflammation

Read this article at
Abstract
Objective
Ongoing neuroaxonal damage is a major contributor to disease progression and long-term disability in multiple sclerosis. However, spatio-temporal distribution and pathophysiological mechanisms of neuroaxonal damage during acute relapses and later chronic disease stages remain poorly understood.
Methods
Here, we applied immunohistochemistry, single-molecule array, spatial transcriptomics, and microglia/axon co-cultures to gain insight into spatio-temporal neuroaxonal damage in experimental autoimmune encephalomyelitis (EAE).
Results
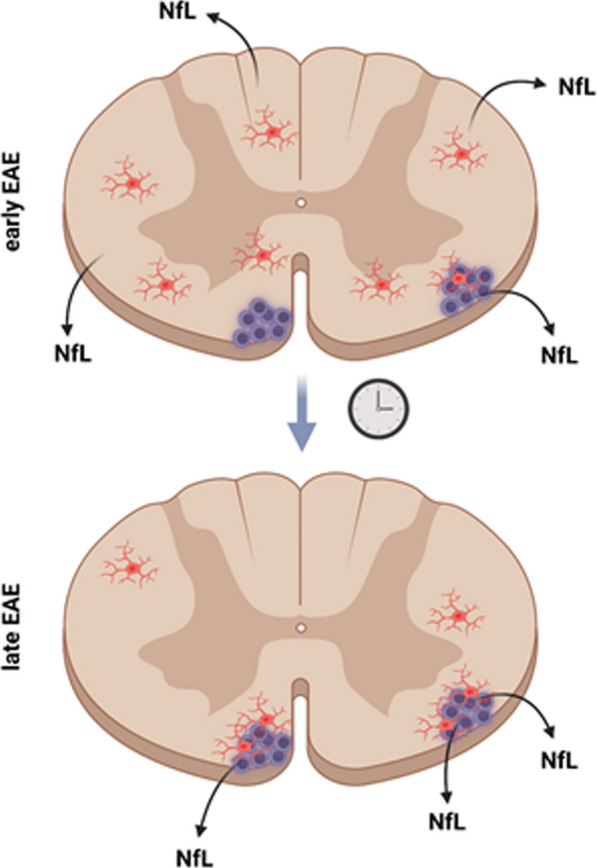
Association of spinal cord white matter lesions and blood-based neurofilament light (sNfL) levels revealed a distinct, stage-dependent anatomical pattern of neuroaxonal damage: in chronic EAE, sNfL levels were predominately associated with anterolateral lumbar lesions, whereas in early EAE sNfL showed no correlation with lesions in any anatomical location. Furthermore, neuroaxonal damage in late EAE was largely confined to white matter lesions but showed a widespread distribution in early EAE. Following this pattern of neuroaxonal damage, spatial transcriptomics revealed a widespread cyto- and chemokine response at early disease stages, whereas late EAE was characterized by a prominent glial cell accumulation in white matter lesions. These findings were corroborated by immunohistochemistry and microglia/axon co-cultures, which further revealed a strong association between CNS myeloid cell activation and neuroaxonal damage both in vivo and in vitro.
Interpretation
Our findings indicate that CNS myeloid cells may play a crucial role in driving neuroaxonal damage in EAE. Moreover, neuroaxonal damage can progress in a stage-dependent centripetal manner, transitioning from normal-appearing white matter to focal white matter lesions. These insights may contribute to a better understanding of neurodegeneration and elevated sNfL levels observed in multiple sclerosis patients at different disease stages.
Related collections
Most cited references45

- Record: found
- Abstract: found
- Article: found
Integrated analysis of multimodal single-cell data

- Record: found
- Abstract: found
- Article: found
clusterProfiler 4.0: A universal enrichment tool for interpreting omics data
- Record: found
- Abstract: found
- Article: not found