- Record: found
- Abstract: found
- Article: not found
In Vivo Imaging of oskar mRNA Transport Reveals the Mechanism of Posterior Localization
Read this article at
Summary
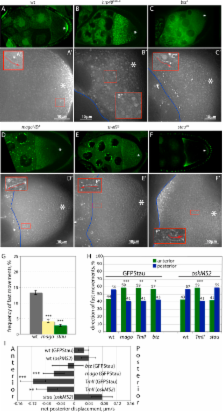
oskar mRNA localization to the posterior of the Drosophila oocyte defines where the abdomen and germ cells form in the embryo. Although this localization requires microtubules and the plus end-directed motor, kinesin, its mechanism is controversial and has been proposed to involve active transport to the posterior, diffusion and trapping, or exclusion from the anterior and lateral cortex. By following oskar mRNA particles in living oocytes, we show that the mRNA is actively transported along microtubules in all directions, with a slight bias toward the posterior. This bias is sufficient to localize the mRNA and is reversed in mago, barentsz, and Tropomyosin II mutants, which mislocalize the mRNA anteriorly. Since almost all transport is mediated by kinesin, oskar mRNA localizes by a biased random walk along a weakly polarized cytoskeleton. We also show that each component of the oskar mRNA complex plays a distinct role in particle formation and transport.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Single mRNA molecules demonstrate probabilistic movement in living mammalian cells.
- Record: found
- Abstract: found
- Article: not found
Oskar organizes the germ plasm and directs localization of the posterior determinant nanos.
- Record: found
- Abstract: found
- Article: not found
