- Record: found
- Abstract: found
- Article: found
Biomarker-Guided Anti-Egfr Rechallenge Therapy in Metastatic Colorectal Cancer
Read this article at
Abstract
Simple Summary
The survival of patients with metastatic colorectal cancer (mCRC) has been improved over the years and now reaches 30–40 months. However, few therapeutic options are available after failure of first- and second-line treatments. In fact, prognosis of chemo-refractory mCRC remains poor. Therefore, new therapeutic strategies are needed. Emerging evidence suggest that retreatment with epidermal growth factor (EGFR) inhibitors after a treatment break, in patients that obtained a clinical benefit by previous anti-EGFR, could lead to prolonged survival. The rationale beyond this “rechallenge” strategy is that after a “treatment holiday” EGFR resistant cancer cells decay, restoring the sensibility to EGFR blockade. In this review we analyze the current knowledge of retreatment with EGFR inhibitors, examine the role of novel biomarkers that can guide the appropriate selection of patients. Finally, we discuss future perspectives and on-going clinical trials.
Abstract
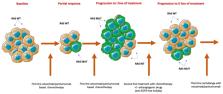
The prognosis of patients with metastatic colorectal cancer (mCRC) who progressed to the first and the second lines of treatment is poor. Thus, new therapeutic strategies are needed. During the last years, emerging evidence suggests that retreatment with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (MAbs) in the third line of mCRC patients, that have previously obtained clinical benefit by first-line therapy with anti-EGFR MAbs plus chemotherapy, could lead to prolonged survival. The rationale beyond this “rechallenge” strategy is that, after disease progression to first line EGFR-based therapy, a treatment break from anti-EGFR drugs results in RAS mutant cancer cell decay, restoring the sensitivity of cancer cells to cetuximab and panitumumab. In fact, rechallenge treatment with anti-EGFR drugs has shown promising clinical activity, particularly in patients with plasma RAS and BRAF wild type circulating tumor DNA, as defined by liquid biopsy analysis at baseline treatment. The aim of this review is to analyze the current knowledge on rechallenge and to investigate the role of novel biomarkers that can guide the appropriate selection of patients that could benefit from this therapeutic strategy. Finally, we discuss on-going trials and future perspectives.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
ESMO consensus guidelines for the management of patients with metastatic colorectal cancer.
- Record: found
- Abstract: found
- Article: not found
Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial
- Record: found
- Abstract: found
- Article: not found