- Record: found
- Abstract: found
- Article: not found
Comparative morphology of the venom apparatus in the braconid wasp subfamily Rogadinae (Insecta, Hymenoptera, Braconidae) and related taxa
Abstract
Zaldivar‐River ón, A., Areekul, B., Shaw, M. R. & Quicke, D. L. J. (2004). Comparative morphology of the venom apparatus in the braconid wasp subfamily Rogadinae (Insecta, Hymenoptera, Braconidae) and related taxa. — Zoologica Scripta, 33, 223–237.
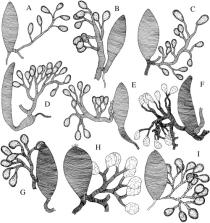
The morphology of the venom apparatus intima in representatives of 38 genera of the problematic braconid wasp subfamily Rogadinae and other cyclostome braconids was investigated and a preliminary phylogenetic analysis for the group was performed with the information obtained. Despite the limited number of characters, the data suggest several relationships at various taxonomic levels. The venom apparatus in the Clinocentrini and the Stiropiini is relatively unmodified and similar to that found in other genera previously placed within a broader concept of the Rogadinae (e.g. genera of Lysitermini, Pentatermini, Tetratermini, Hormiini) and also to that of the Betylobraconinae. The presence of a cone of filaments located inside the secondary venom duct near to its insertion on the venom reservoir/primary venom duct is proposed as a synapomorphy for the tribe Rogadini to the exclusion of Stiropiini, Clinocentrini and Yeliconini. Other features of the secondary venom duct and its insertion on the venom reservoir/primary venom duct support a number of relationships between the genera of the Rogadini and also within the large genus Aleiodes. A clade containing 15 Rogadini genera ( Bathoteca, Bathotecoides, Bulborogas, Canalirogas, Colastomion, Conspinaria, Cystomastacoides, Macrostomion, Megarhogas, Myocron, Pholichora, Rectivena, Rogas, Spinaria and Triraphis) is supported by the presence of a thickened and short secondary venom duct, whereas the different members of Aleiodes (excluding members of the subgenus Heterogamus) and Cordylorhogas are distinguished by having a recessed secondary venom duct with well‐defined and numerous internal filaments. New World Rogas species exhibit a unique venom apparatus and may not be closely related to the Old World ones. Features of the venom apparatus of the enigmatic genus Telengaia and the exothecine genera Shawiana and Colastes suggest that the Telengainae and Exothecinae are both closely related to the Braconinae, Gnamptodontinae, and possibly to the Opiinae and Alysiinae. An unsculptured venom reservoir was found in one specimen of the type species of Avga, A. choaspes, which is consistent with it occupying either a very basal position within the cyclostome braconids or belonging to a recently recognized ‘Gondwanan’ clade that also includes the Aphidiinae.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
A Successive Approximations Approach to Character Weighting
- Record: found
- Abstract: not found
- Article: not found
Ovipositor structure and relationships within the Hymenoptera, with special reference to the Ichneumonoidea
- Record: found
- Abstract: found
- Article: not found
