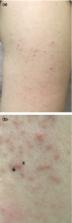
To the Editor: Baden et al. 1 report on a phase 3 clinical trial of the mRNA-1273 vaccine against SARS-CoV-2, and they provide information on immediate injection-site reactions, which were observed in 84.2% of the participants after the first dose. The trial also showed that delayed injection-site reactions (defined in that trial as those with an onset on or after day 8) occurred in 244 of the 30,420 participants (0.8%) after the first dose and in 68 participants (0.2%) after the second dose. These reactions included erythema, induration, and tenderness. The reactions typically resolved over the following 4 to 5 days. However, these reactions were not further characterized, and links between reactions after the first dose and those after the second dose were not provided to inform clinical care. We have also observed delayed large local reactions to the mRNA-1273 vaccine, with a median onset on day 8 (range, 4 to 11) after the first dose. These reactions had a variable appearance (Figure 1). Here, we report on a series of 12 patients with these reactions, all of which appeared near the injection site after complete resolution of the initial local and systemic symptoms associated with vaccination. Five of the reactions were grade 3 plaques (≥10 cm in diameter) (Table 1). Some patients had concurrent systemic adverse effects, and among these patients, 2 had additional skin findings. Most patients received treatment for their symptoms (e.g., with ice and antihistamines). Some patients received glucocorticoids (topical, oral, or both), and 1 patient received antibiotic therapy for presumptive cellulitis. The symptoms resolved a median of 6 days after onset (range, 2 to 11). Our suspicion of delayed-type or T-cell–mediated hypersensitivity was supported by skin-biopsy specimens obtained from a patient with a delayed large local reaction who was not among the 12 patients described here. Those specimens showed superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells (see Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Given that neither local injection-site reactions nor delayed-type hypersensitivity reactions are contraindications to subsequent vaccination, 2 all 12 patients were encouraged to receive the second dose and completed their mRNA-1273 vaccination course. Although half the patients did not have a recurrence of large local reactions, three patients had recurrent reactions that were similar to those after the initial dose, and three patients had recurrent reactions that were of a lower grade than those after the initial dose. The median onset of cutaneous symptoms after the second dose (day 2; range, 1 to 3) was earlier than that after the first dose (Table 1). Clinicians may not be prepared to address delayed local reactions to the mRNA-1273 vaccine. Given the scale-up of mass vaccination campaigns across the world, these reactions are likely to generate concerns among patients and requests for evaluation. These reactions have not been consistently recognized, guidance regarding the second dose of vaccine has varied, and many patients have unnecessarily received antibiotic agents. We hope this letter encourages additional reporting and communication regarding the epidemiologic characteristics, causes, and implications of these delayed cutaneous reactions, since this information might allay the concerns of patients, encourage completion of vaccination, and minimize the unnecessary use of antibiotic agents.