- Record: found
- Abstract: found
- Article: found
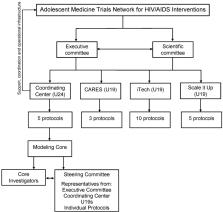
Model-Based Methods to Translate Adolescent Medicine Trials Network for HIV/AIDS Interventions Findings Into Policy Recommendations: Rationale and Protocol for a Modeling Core (ATN 161)
Abstract
Background
The United States Centers for Disease Control and Prevention estimates that approximately 60,000 US youth are living with HIV. US youth living with HIV (YLWH) have poorer outcomes compared with adults, including lower rates of diagnosis, engagement, retention, and virologic suppression. With Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) support, new trials of youth-centered interventions to improve retention in care and medication adherence among YLWH are underway.
Objective
This study aimed to use a computer simulation model, the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-Adolescent Model, to evaluate selected ongoing and forthcoming ATN interventions to improve viral load suppression among YLWH and to define the benchmarks for uptake, effectiveness, durability of effect, and cost that will make these interventions clinically beneficial and cost-effective.
Methods
This protocol, ATN 161, establishes the ATN Modeling Core. The Modeling Core leverages extensive data—already collected by successfully completed National Institutes of Health–supported studies—to develop novel approaches for modeling critical components of HIV disease and care in YLWH. As new data emerge from ongoing ATN trials during the award period about the effectiveness of novel interventions, the CEPAC-Adolescent simulation model will serve as a flexible tool to project their long-term clinical impact and cost-effectiveness. The Modeling Core will derive model input parameters and create a model structure that reflects key aspects of HIV acquisition, progression, and treatment in YLWH. The ATN Modeling Core Steering Committee, with guidance from ATN leadership and scientific experts, will select and prioritize specific model-based analyses as well as provide feedback on derivation of model input parameters and model assumptions. Project-specific teams will help frame research questions for model-based analyses as well as provide feedback regarding project-specific inputs, results, sensitivity analyses, and policy conclusions.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6.
- Record: found
- Abstract: found
- Article: not found
Expanded screening for HIV in the United States--an analysis of cost-effectiveness.
- Record: found
- Abstract: found
- Article: not found