- Record: found
- Abstract: found
- Article: found
Tenofovir versus entecavir on the prognosis of hepatitis B-related hepatocellular carcinoma after surgical resection: a randomised controlled trial
Read this article at
Abstract
Background:
Nucleot(s)ide analog treatment (entecavir (ETV) and tenofovir (TDF)) is reported to be associated with decreased tumor recurrence and death in hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) patients, yet further work is needed to evaluate the different efficacies of these two agents on the prognosis of early-stage HBV-related HCC patients after curative liver resection.
Material and methods:
From July 2017 to January 2019, 148 patients with HBV-related HCC who underwent curative liver resection were randomized to receive TDF ( n=74) or ETV ( n=74) therapy. The primary end point was tumor recurrence in the intention-to-treat population. Overall survival and tumor recurrence of patients were compared by multivariable-adjusted Cox regression and competing risk analyses.
Results:
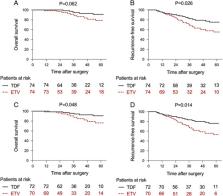
During the follow-up with continued antiviral therapy, 37 (25.0%) patients developed tumor recurrence, and 16 (10.8%) patients died ( N=15) or received liver transplantation ( N=1). In the intention-to-treat cohort, the recurrence-free survival for the TDF group was significantly better than that for the ETV group ( P=0.026). In the multivariate analysis, the relative risks of recurrence and death/liver transplantation for ETV therapy were 3.056 (95% CI: 1.015–9.196; P=0.047) and 2.566 (95% CI: 1.264–5.228; P=0.009), respectively. Subgroup analysis of the PP population indicated a better overall survival and RFS of patients receiving TDF therapy ( P=0.048; hazard ratio (HR) =0.362; 95% CI: 0.132–0.993 and P=0.014; HR =0.458; 95% CI: 0.245–0.856). Additionally, TDF therapy was an independent protective factor against late tumor recurrence ( P=0.046; (HR)=0.432; 95% CI: 0.189–0.985) but not against early tumor recurrence ( P=0.109; HR =1.964; 95% CI: 0.858–4.494).
Related collections
Most cited references27
- Record: found
- Abstract: not found
- Article: not found
EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma
- Record: found
- Abstract: found
- Article: not found
Hepatocellular carcinoma

- Record: found
- Abstract: found
- Article: not found