- Record: found
- Abstract: found
- Article: found
LPHN3, a presynaptic adhesion-GPCR implicated in ADHD, regulates the strength of neocortical layer 2/3 synaptic input to layer 5
Read this article at
Abstract
Background
Latrophilins (LPHNs) are a small family of neuronal adhesion-GPCRs originally discovered as receptors for the black widow spider toxin α-latrotoxin. Mutations in LPHN3 have recently been identified as risk factors for attention deficit hyperactivity disorder (ADHD) in humans, but their physiological function has remained elusive. In this study, we tested two hypotheses regarding LPHN3 function: (1) LPHN3 regulates synaptic transmission by modulating probability of release; and (2) LPHN3 controls synapse development and the abundance of synapses.
Results
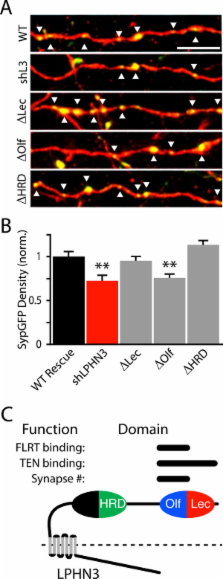
We manipulated LPHN3 expression in mouse layer 2/3 (L2/3) pyramidal neurons and examined the consequences on the L2/3 to L5 cortical microcircuit. Employing an optogenetic strategy combined with shRNA knockdown of LPHN3, we found that LPHN3 did not influence probability of release at synapses formed by L2/3 neurons onto L5 pyramidal cells. The strength of L2/3 afferent input to L5, however, was weakened by loss of LPHN3. Using Synaptophysin-GFP as an anatomical marker of presynaptic terminals, we found that the density of synapses formed by L2/3 axons in L5 was reduced when LPHN3 was lost. Finally, we investigated the structural organization of the extracellular domain of LPHN3. We used single particle negative stain electron microscopy to image the extracellular domain of LPHN3 and showed that the Olfactomedin and Lectin domains form a globular domain on an elongated stalk. Cell-based binding experiments with mutant proteins revealed that the Olfactomedin domain was required for binding to FLRT3, whereas both the Olfactomedin and Lectin domains were involved in binding to Teneurin 1. Mutant LPHN3 lacking the Olfactomedin domain was not capable of rescuing the deficit in presynaptic density following knockdown of endogenous LPHN3.
Conclusions
We find that LPHN3 regulates the number of synapses formed by L2/3 neurons in L5 and the strength of synaptic drive from the L2/3-L5 pathway. The Olfactomedin domain of LPHN3 is required for this effect on synapse number and binding to its postsynaptic ligand FLRT3. We propose that LPHN3 functions in synaptic development and is important in determining the connectivity rates between principal neurons in the cortex.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Negative Staining and Image Classification – Powerful Tools in Modern Electron Microscopy
- Record: found
- Abstract: found
- Article: found
Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities.
- Record: found
- Abstract: found
- Article: not found