- Record: found
- Abstract: found
- Article: found
Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury
Read this article at
Abstract
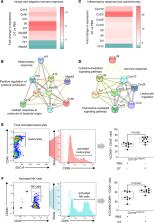
Macrophages are commonly thought to contribute to the pathophysiology of preterm labor by amplifying inflammation — but a protective role has not previously been considered to our knowledge. We hypothesized that given their antiinflammatory capability in early pregnancy, macrophages exert essential roles in maintenance of late gestation and that insufficient macrophages may predispose individuals to spontaneous preterm labor and adverse neonatal outcomes. Here, we showed that women with spontaneous preterm birth had reduced CD209 +CD206 + expression in alternatively activated CD45 +CD14 +ICAM3 – macrophages and increased TNF expression in proinflammatory CD45 +CD14 +CD80 +HLA-DR + macrophages in the uterine decidua at the materno-fetal interface. In Cd11b DTR/DTR mice, depletion of maternal CD11b + myeloid cells caused preterm birth, neonatal death, and postnatal growth impairment, accompanied by uterine cytokine and leukocyte changes indicative of a proinflammatory response, while adoptive transfer of WT macrophages prevented preterm birth and partially rescued neonatal loss. In a model of intra-amniotic inflammation–induced preterm birth, macrophages polarized in vitro to an M2 phenotype showed superior capacity over nonpolarized macrophages to reduce uterine and fetal inflammation, prevent preterm birth, and improve neonatal survival. We conclude that macrophages exert a critical homeostatic regulatory role in late gestation and are implicated as a determinant of susceptibility to spontaneous preterm birth and fetal inflammatory injury.
Abstract

Related collections
Most cited references102

- Record: found
- Abstract: found
- Article: found
STRING v10: protein–protein interaction networks, integrated over the tree of life
- Record: found
- Abstract: found
- Article: not found
Epidemiology and causes of preterm birth

- Record: found
- Abstract: found
- Article: found