- Record: found
- Abstract: found
- Article: found
Ultra-high density electrodes improve detection, yield, and cell type specificity of brain recordings
Read this article at
Abstract
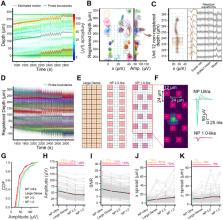
To study the neural basis of behavior, we require methods to sensitively and accurately measure neural activity at single neuron and single spike resolution. Extracellular electrophysiology is a principal method for achieving this, but it has biases in the neurons it detects and it imperfectly resolves their action potentials. To overcome these limitations, we developed a silicon probe with significantly smaller and denser recording sites than previous designs, called Neuropixels Ultra (NP Ultra). This device measures neuronal activity at ultra-high densities (>1300 sites per mm, 10 times higher than previous probes), with 6 μm center-to-center spacing and low noise. This device effectively comprises an implantable voltage-sensing camera that captures a planar image of a neuron’s electrical field. We introduce a new spike sorting algorithm optimized for these probes and use it to find that the yield of visually-responsive neurons in recordings from mouse visual cortex improves ~3-fold. Recordings across multiple brain regions and four species revealed a subset of unexpectedly small extracellular action potentials not previously reported. Further experiments determined that, in visual cortex, these do not correspond to major subclasses of interneurons and instead likely reflect recordings from axons. Finally, using ground-truth identification of cortical inhibitory cell types with optotagging, we found that cell type was discriminable with approximately 75% success among three types, a significant improvement over lower-resolution recordings. NP Ultra improves spike sorting performance, sampling bias, and cell type classification.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes.
- Record: found
- Abstract: found
- Article: not found
Fully integrated silicon probes for high-density recording of neural activity
- Record: found
- Abstract: found
- Article: not found
