- Record: found
- Abstract: found
- Article: found
The impact of gender and sex on diagnosis, treatment outcomes and health-related quality of life in patients with axial spondyloarthritis
Read this article at
Abstract
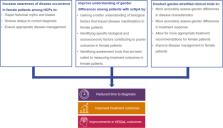
Axial spondyloarthritis (axSpA) is a chronic inflammatory rheumatic condition, historically considered a predominantly male disease. However, increasing evidence suggests a more equal prevalence between men and women. Of the limited research conducted to date, it is apparent that gender differences exist in terms of time to diagnosis, treatment outcomes and health-related quality of life (HRQoL). Despite this, women are underrepresented in clinical trials and most studies do not stratify by gender to identify potential differences in terms of disease manifestations and treatment response. In this perspectives article, we reflect on the potential biological and social factors contributing to these differences and propose three key areas of education and research that should be prioritised in order to address the unmet needs of female patients with axSpA, namely: (1) to identify ways to increase awareness of disease occurrence in female patients among healthcare professionals (HCPs), (2) to improve understanding of gender differences in disease manifestation and outcomes, and (3) to conduct gender-stratified clinical trials with a representative sample of female patients.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection.
- Record: found
- Abstract: found
- Article: found
2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis
- Record: found
- Abstract: found
- Article: not found