- Record: found
- Abstract: found
- Article: found
The efficacy and safety of immunotherapy as first−line treatment for extensive-stage small cell lung cancer: evaluating based on reconstructed individual patient data
Read this article at
Abstract
Objective
Selecting between programmed cell death ligand 1 (PD-L1) inhibitor or programmed cell death 1 (PD-1) inhibitor plus chemotherapy as first-line treatment for extensive-stage small cell lung cancer (ES-SCLC) patients urgently needs to be answered.
Methods
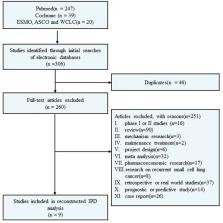
Eligible phase 3 randomized clinical trials evaluating regimens based on PD-1/PD-L1 inhibitors as first-line treatment in ES-SCLC patients were systematically searched on the PubMed and Cochrane Library databases and major international conferences from 01/01/2018 to 18/09/2023. The individual patient data (IPD) were recuperated from the Kaplan–Meier curves of the overall survival (OS) and progression-free survival (PFS) of the included studies using the IPDfromKM method. The reconstructed data were pooled into unified arms, including the PD-L1 inhibitor plus chemotherapy group (PD-L1 group), PD-1 inhibitor plus chemotherapy group (PD-1 group), and PD-1 (L1) inhibitor and chemotherapy plus other (anlotinib group, tiragolumab group, and tremelimumab group). Subsequently, the PD-L1 group was indirectly compared with the other groups. A standard statistical analysis was conducted using the “survival” package for the time-to-event endpoint. The primary outcomes were the OS and PFS of the PD-L1 group and the PD-1 inhibitor group. The secondary outcomes included safety and the 12- and 24-month restricted mean survival time (RMST) of the PD-L1 group and PD-1 group.
Results
A total of 9 studies including 11 immunotherapy cohorts were included. No significant difference in PFS (hazard ratio [HR]: 0.96, 95% confidence interval [CI]: 0.86–1.06), OS (HR: 0.94, 95% CI: 0.84–1.05), and 12-month and 24-month RMST for OS (P = 0.198 and P = 0.216, respectively) was observed between the PD-L1 group and the PD-1 group. In contrast, the anlotinib group showed significantly better OS (HR: 0.70, 95% CI: 0.55–0.89), PFS (HR: 0.69, 95% CI: 0.58–0.83), and RMST for OS compared to the PD-L1 group. The tiragolumab group showed similar efficacy to the PD-L1 group. However, the tremelimumab group exhibited inferior efficacy than the PD-L1 group. The incidence of ≥grade 3 treatment-emergent adverse events (TEAEs) was significantly higher in the PD-1 group compared to the PD-L1 group (85.4% vs. 69.6%, P <.001), whereas the incidence of irAEs was similar between the two groups.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer
- Record: found
- Abstract: found
- Article: not found
Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation
- Record: found
- Abstract: found
- Article: not found