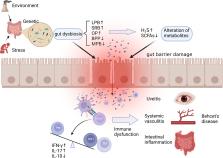
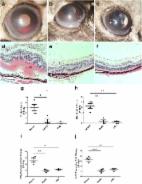
Background Behcet’s disease (BD) is a recalcitrant, multisystemic inflammatory disease that can lead to irreversible blindness. Microbial agents have been considered to contribute to the pathogenesis of this disease, but the underlying mechanisms remain unclear. In this study, we investigated the association of gut microbiome composition with BD as well as its possible roles in the development of this disease. Methods Fecal and saliva samples were collected from 32 active BD patients and 74 healthy controls. DNA extracted from fecal samples was subjected to metagenomic analysis, whereas DNA extracted from saliva samples was subjected to 16S rRNA gene sequencing analysis. The results were used to compare the composition and biological function of the microbiome between patients and healthy controls. Lastly, transplantation of pooled fecal samples from active BD patients into B10RIII mice undergoing experimental autoimmune uveitis (EAU) was performed to determine the causal relationship between the gut microbiome and BD. Results Fecal samples from active BD patients were shown to be enriched in Bilophila spp., a sulfate-reducing bacteria (SRB) and several opportunistic pathogens (e.g., Parabacteroides spp. and Paraprevotella spp.) along with a lower level of butyrate-producing bacteria (BPB) Clostridium spp. and methanogens (Methanoculleus spp. Methanomethylophilus spp.). Analysis of microbial functions revealed that capsular polysaccharide transport system, oxidation-reduction process, type III, and type IV secretion systems were also increased in active BD patients. Network analysis showed that the BD-enriched SRB and opportunistic pathogens were positively correlated with each other, but they were negatively associated with the BPB and methanogens. Animal experiments revealed that fecal microbiota transplantation with feces from BD patients significantly exacerbated EAU activity and increased the production of inflammatory cytokines including IL-17 and IFN-γ. Conclusions Our findings revealed that BD is associated with considerable gut microbiome changes, which is corroborated by a mouse study of fecal microbiota transplants. A model explaining the association of the gut microbiome composition with BD pathogenesis is proposed. Electronic supplementary material The online version of this article (10.1186/s40168-018-0520-6) contains supplementary material, which is available to authorized users.