- Record: found
- Abstract: found
- Article: found
Adherence to direct oral anticoagulant treatment for atrial fibrillation in the Netherlands: A surveillance study
Read this article at
Abstract
Background
Adherence to direct oral anticoagulants (DOACs) in patients with atrial fibrillation in every day practice may be less than in clinical trials.
Aims
To assess adherence to DOACs in atrial fibrillation patients in every day practice and identify predictors for non‐adherence.
Methods
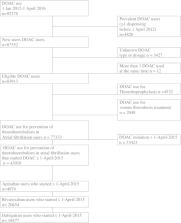
Individual linked dispensing data of atrial fibrillation patients who used DOACs were obtained from the Foundation for Pharmaceutical Statistics covering the Netherlands between 2012 and 2016. One year adherence to DOAC was calculated for initial DOAC as proportion of days covered (PDC) ≥80% and the association between clinical variables and adherence was assessed using logistic regression. In addition, we measured non‐persistence, that is, patients who completely stopped their initial DOAC within 1 year follow‐up.
Results
A total of 4797 apixaban‐, 20 454 rivaroxaban‐ and 18 477 dabigatran users were included. The mean age was 69 years (n = 43 910), which was similar for the DOAC types. The overall proportion of patients with PDC ≥80% was 76%, which was highest for apixaban‐ (87%), followed by dabigatran‐ (80%) and rivaroxaban (69%) users. Multivariable analyses revealed that age ≤60 years, no concomitant drug use were predictors for non‐adherence. Of atrial fibrillation patients who continued treatment, 97% had a PDC ≥80%, compared with only 56% for those who discontinued their DOAC treatment within 1 year.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Adherence to Medication
- Record: found
- Abstract: found
- Article: not found
Medication compliance and persistence: terminology and definitions.
- Record: found
- Abstract: not found
- Article: not found
2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.