- Record: found
- Abstract: found
- Article: found
Kinase Inhibitors of DNA-PK, ATM and ATR in Combination with Ionizing Radiation Can Increase Tumor Cell Death in HNSCC Cells While Sparing Normal Tissue Cells
Read this article at
Abstract
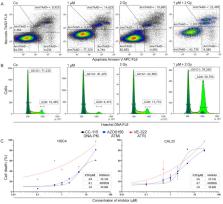
(1) Kinase inhibitors (KI) targeting components of the DNA damage repair pathway are a promising new type of drug. Combining them with ionizing radiation therapy (IR), which is commonly used for treatment of head and neck tumors, could improve tumor control, but could also increase negative side effects on surrounding normal tissue. (2) The effect of KI of the DDR (ATMi: AZD0156; ATRi: VE-822, dual DNA-PKi/mTORi: CC-115) in combination with IR on HPV-positive and HPV-negative HNSCC and healthy skin cells was analyzed. Cell death and cell cycle arrest were determined using flow cytometry. Additionally, clonogenic survival and migration were analyzed. (3) Studied HNSCC cell lines reacted differently to DDRi. An increase in cell death for all of the malignant cells could be observed when combining IR and KI. Healthy fibroblasts were not affected by simultaneous treatment. Migration was partially impaired. Influence on the cell cycle varied between the cell lines and inhibitors; (4) In conclusion, a combination of DDRi with IR could be feasible for patients with HNSCC. Side effects on healthy cells are expected to be limited to normal radiation-induced response. Formation of metastases could be decreased because cell migration is impaired partially. The treatment outcome for HPV-negative tumors tends to be improved by combined treatment.
Related collections
Most cited references65

- Record: found
- Abstract: found
- Article: found
Cancer and Radiation Therapy: Current Advances and Future Directions
- Record: found
- Abstract: found
- Article: not found