- Record: found
- Abstract: found
- Article: not found
A randomized, double-blind, placebo-controlled trial of intravenous alpha-1 antitrypsin for acute respiratory distress syndrome secondary to COVID-19
Read this article at
Abstract
Background
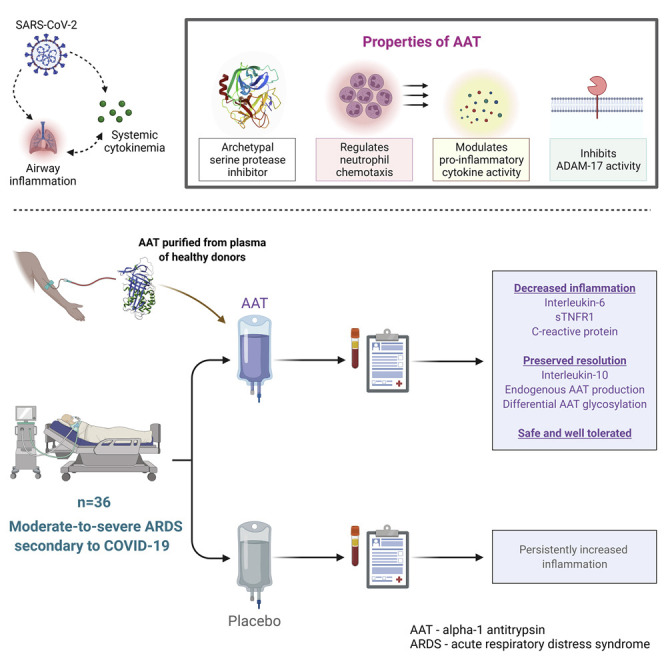
Patients with severe COVID-19 develop a febrile pro-inflammatory cytokinemia with accelerated progression to acute respiratory distress syndrome (ARDS). Here we report the results of a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of intravenous plasma-purified alpha-1 antitrypsin (AAT) for moderate-to-severe ARDS secondary to COVID-19 (EudraCT 2020-001391-15).
Methods
Patients (n=36) were randomized to receive weekly placebo, weekly AAT (Prolastin, Grifols, S.A.; 120mg/kg), or AAT once followed by weekly placebo. The primary endpoint was the change in plasma interleukin (IL)-6 concentration at one week. In addition to assessing safety and tolerability, changes in plasma levels of IL-1β, IL-8, IL-10 and soluble TNF receptor 1 and clinical outcomes were assessed as secondary endpoints.
Findings
Treatment with IV AAT resulted in decreased inflammation and was safe and well tolerated. The study met its primary endpoint, with decreased circulating IL-6 concentrations at one week in the treatment group. This was in contrast to the placebo group, where IL-6 was increased. Similarly, plasma sTNFR1 was substantially decreased in the treatment group while remaining unchanged in patients receiving placebo. IV AAT did not definitively reduce levels of IL-1β, IL-8 and IL-10. No difference in mortality or ventilator-free days was observed between groups, though a trend towards decreased time-on-ventilator was observed in AAT-treated patients.
Graphical Abstract
Abstract
McElvaney and colleagues present the results of a randomized placebo-controlled trial of alpha-1 antitrypsin (AAT) for patients with acute respiratory distress syndrome secondary to COVID-19. Circulating levels of interleukin-6 and other pro-inflammatory mediators were decreased at one week in the treatment group, identifying a potential anti-inflammatory therapeutic for critical illness.