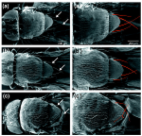
Background Ribosomes are sophisticated macromolecular machines that catalyze cellular protein synthesis in all cells of all organisms. They have an ancient evolutionary origin and are essential for cell growth, proliferation and viability. Though larger and more complex in higher organisms, both the structure and function of ribosomes have been conserved throughout evolution. Genetic approaches in Drosophila melanogaster have shown that disrupting ribosome function can result in an array of fascinating dominant phenotypes [1,2]. Despite this, there has so far been no comprehensive inventory of genes encoding ribosome components in this organism, nor any systematic effort to determine their mutant phenotypes. All ribosomes comprise a set of ribosomal proteins (RPs) surrounding a catalytic core of ribosomal RNA (rRNA). Bacteria possess a single type of ribosome composed of three rRNA molecules and typically 54 RPs. All eukaryotic cells, in contrast, contain at least two distinct types of ribosomes: cytoplasmic ribosomes (cytoribosomes) and mitochondrial ribosomes (mitoribosomes). Cytoribosomes are found on the endoplasmic reticulum and in the aqueous cytoplasm. They translate all mRNAs produced from nuclear genes and perform the vast majority of cellular protein synthesis. Each cytoribosome contains four different rRNAs and 78-80 cytoplasmic RPs (CRPs). Mitoribosomes consist of only two rRNA molecules and up to 80 mitochondrial RPs (MRPs). They are located in the mitochondrial matrix and synthesize proteins involved in oxidative phosphorylation encoded by those few genes retained in the mitochondrial genome. A third unique type of eukaryotic ribosome is found within the plastids (for example, chloroplasts) of plant and various algal cells. In all cases, distinct small and large ribosomal subunits exist that join together during the translation initiation process to form mature ribosomes capable of protein synthesis. (See references [3-6] for general reviews of ribosomal structure and function.) The protein components of ribosomes are interesting from several points of view. First, and most obviously, RPs play critical roles in ribosome assembly and function [7]. Second, several RPs perform important extra-ribosomal functions, including roles in DNA repair, transcriptional regulation and apoptosis [6,8]. Third, misexpression of human CRP and MRP genes has been implicated in a wide spectrum of human syndromes and diseases, including Diamond-Blackfan anaemia [9], Turner syndrome [10], hearing loss [11] and cancer [12]. Fourth, mutations in the CRP genes of D. melanogaster are important tools for the study of growth, development and cell competition [2]. Finally, many RPs are conserved from bacteria to humans, so their peptide and nucleotide sequences are useful for studying phylogenetic relationships [13]. The first eukaryotic CRPs characterized in detail were isolated from the rat cytoribosome [3]. Individual proteins were separated by two-dimensional gel electrophoresis and named from their origin in the small (S) or large (L) subunit and their relative electrophoretic migration positions, for example, RPS9 or RPL28. Subsequent studies revealed that some protein spots contained non-ribosomal proteins or chemically modified versions of another CRP, and that some spots contained two co-migrating CRPs [3,5]. Consequently, the nomenclature system used today contains numerical gaps as well as 'A' suffixes for those additional CRPs not resolved by the original electrophoresis (for example, RPL36A). Seventy-nine distinct mammalian CRPs are now acknowledged and their amino acid sequences and biochemical properties have been described [5,14]. With the exception of RPLP1 and RPLP2, each of which forms homodimers in the cytoribosomal large subunit [15], all CRPs are present as single molecules in each cytoribosome [3]. Seventy-eight different mammalian MRPs have been described [6] and their individual amino acid sequences and biochemical properties have been determined [16,17]. Although the nomenclature of MRPs was originally based on electrophoretic properties, the current system reflects homology between mammalian MRPs and their bacterial orthologs [18]. Thus, MRPS1 through MRPS21 are orthologous to Escherichia coli RPs S1-S21, while higher numbers have been assigned to the MRPs not found in bacteria. Gaps also exist in MRP numbering because a gap occurs in the bacterial enumeration or because there is no mammalian ortholog. The RPs of D. melanogaster were first studied in the 1970s and early 1980s. Up to 78 individual CRPs were observed on two-dimensional gels [19-31] and about 30 were purified and analyzed biochemically [32,33]. A more recent characterization used mass spectrometry to identify 52 D. melanogaster CRPs [34], all of which are orthologous to known mammalian CRPs. The protein composition of Drosophila mitoribosomes has not been characterized biochemically to date. CRPs and MRPs are encoded by the nuclear genome. Knowledge of the primary sequences of rat CRPs and bovine MRPs has led to the identification and mapping of the RP-encoding genes in many eukaryotic species [14]. Indeed, systematic analyses of whole RP gene sets have been described for several organisms, including Saccharomyces cerevisiae [35], Arabidopsis thaliana [36] and humans [37-40]. However, the complete set of D. melanogaster CRP and MRP genes has not been previously documented or characterized. Several D. melanogaster RP genes were initially identified by virtue of their dominant 'Minute' mutant phenotypes [2], which include prolonged development, low fertility and viability, altered body size and abnormally short, thin bristles on the adult body. All of these phenotypes may be explained by a cell-autonomous defect in protein biosynthesis: the production of each bristle, for example, requires a very high rate of protein synthesis in a single cell during a short developmental period. Merriam and colleagues reported the first unequivocal molecular link between a Minute locus and a CRP gene in 1985 [41]. Since then, 14 additional Minute loci have been definitively linked with distinct CRP genes [2,42-53]. However, there are at least 35 genetically validated Minute loci that have not yet been associated with a specific gene and there may be additional Minute genes to be discovered. Several investigators have hypothesized that all Minute loci encode protein components of ribosomes (reviewed in [2]). Whether this is truly the case and whether both CRP and MRP genes are associated with Minute phenotypes are open and intriguing questions. Many Minute loci were originally identified from the phenotypes of flies heterozygous for a chromosomal deletion [54,55] and all Minute point mutations studied in depth have been found to be loss-of-function alleles [2]. This indicates that Minute phenotypes can be attributed to genetic haploinsufficiency; that is, a single gene copy is not sufficient for normal development. (Note that X-linked mutations that cause Minute phenotypes in heterozygous females are lethal in hemizygous males.) The most popular explanation for the haploinsufficiency of Minute loci is that they correspond to RP genes and that RPs are required in equimolar amounts: halving the copy number of a single RP gene limits the availability of the encoded RP, thereby reducing the number of functional ribosomes that are assembled in the cell and impairing protein synthesis [2]. While this idea is consistent with the available data, there may be other explanations. The reduced fertility and viability associated with many Minute loci makes the recovery of deletions uncovering them rather difficult - the mutant strains are too weak to maintain as stable heterozygous stocks. In fact, some Minute loci are known only from the phenotypes of transient aneuploids [54,56]. This means that several chromosomal regions containing a Minute locus are not uncovered by current deletion collections [57]. This is frustrating for researchers because deletions are basic tools for mutational analysis and are widely used for mapping new mutations and identifying genetic modifiers. Efforts to maximize deletion coverage of the D. melanogaster genome would benefit from a systematic assessment of the relationship between RP genes and Minute loci. It would allow the isolation of deletions that flank haploinsufficient RP genes as closely as possible, or the design of transgenic constructs or chromosomal duplications to rescue the haploinsufficiency of deletions uncovering Minute genes. Here, we report the systematic identification, naming and characterization of all the CRP and MRP genes of D. melanogaster. We have used this information, together with phenotypic data obtained from examining mutation and deficiency strains, to assess the correspondence between RP genes and Minute loci. We find that 66 of the 88 CRP genes identified are, or are very likely to be, haploinsufficient and associated with a Minute phenotype, whereas MRP genes and the remaining 22 CRP genes are not. Significantly, we show that all but one of the known Minute loci in the genome correspond to CRP genes - the single exception encodes a subunit of an essential translation initiation factor. Together, these results identify the majority of haploinsufficient loci in the D. melanogaster genome that significantly affect viability, fertility and/or external morphology, and also provide a mechanistic framework for understanding the Minute syndrome and the phenotypic effects of aneuploidy. Results Identification of D. melanogaster ribosomal protein genes In order to conduct an exhaustive survey of Drosophila CRP and MRP genes, we first performed a series of BLAST searches using human RP sequences as queries, because both CRPs and MRPs have been well-characterized in humans [5,6]. Tables 1 and 2 list the genes we identified together with their cytological locations. Where necessary, D. melanogaster genes were named or renamed according to the standard metazoan RP gene nomenclature proposed by Wool and colleagues [5,58,59] and approved by the HUGO Gene Nomenclature Committee [18], whilst still conforming to FlyBase [60] conventions - that is, CRP genes are given an 'Rp' prefix and MRP genes have an 'mRp' prefix. The seven exceptions to this standard RP nomenclature are mostly genes originally named to reflect a mutant phenotype, for example, the string of pearls (sop) gene encodes RpS2 [61] and bonsai encodes mRpS15 [62,63]. In these cases, the original gene symbol has been preserved, with the apposite RP symbol given as a synonym. Table 1 The CRP genes of D. melanogaster D. melanogaster gene Human CRP Symbol* CG number Location† BLAST E value‡ RPSA sta/RpSA CG14792 X: 2B1 3e-94 RPS2 sop/RpS2 CG5920 2L: 30E1 e-118 RPS3 RpS3 CG6779 3R: 94E13 e-106 RPS3A RpS3A CG2168 4: 101F1 e-100 RPS4 RpS4 CG11276 3L: 69F6 e-121 RPS5 RpS5a CG8922 X: 15E5-7 1e-98 RpS5b CG7014 3R: 88D6 3e-96 RPS6 RpS6 CG10944 X: 7C2 e-103 CG11386 CG11386 X: 7C2 2e-15 CG33222 CG33222 X: 7C2 2e-15 RPS7 RpS7 CG1883 3R: 99E2 1e-74 RPS8 RpS8 CG7808 3R: 99C4 1e-82 RPS9 RpS9 CG3395 3L: 67B11 8e-92 RPS10 RpS10a CG12275 3R: 98A14 4e-43 RpS10b CG14206 X: 18D3 8e-52 RPS11 RpS11 CG8857 2R: 48E8-9 1e-58 RPS12 RpS12 CG11271 3L: 69F5 2e-43 RPS13 RpS13 CG13389 2L: 29B2 2e-74 RPS14 RpS14a CG1524 X: 7C6-7 3e-71 RpS14b CG1527 X: 7C8 3e-71 RPS15 RpS15 CG8332 2R: 53C8 6e-62 RPS15A RpS15Aa CG2033 X: 11E11-12 2e-65 RpS15Ab CG12324 2R: 47C1 6e-65 RPS16 RpS16 CG4046 2R: 58F1 2e-69 RPS17 RpS17 CG3922 3L: 67B5 5e-52 RPS18 RpS18 CG8900 2R: 56F11 2e-69 RPS19 RpS19a CG4464 X: 14F4 5e-48 RpS19b CG5338 3R: 95C13 1e-43 RPS20 RpS20 CG15693 3R: 93A1 7e-50 RPS21 oho23B/RpS21 CG2986 2L: 23B6 3e-30 RPS23 RpS23 CG8415 2R: 50E4 8e-70 RPS24 RpS24 CG3751 2R: 58F3 7e-55 RPS25 RpS25 CG6684 3R: 86D8 1e-38 RPS26 RpS26 CG10305 2L: 36F4 3e-47 RPS27 RpS27 CG10423 3R: 96C8 2e-39 RPS27A RpS27A CG5271 2L: 31E1 8e-80 RPS28 RpS28a CG15527 3R: 99D2 1e-21 RpS28b CG2998 X: 8E7 2e-23 RpS28-like CG34182 2L: 30B3 1e-07 RPS29 RpS29 CG8495 3R: 85E8 5e-23 RPS30 RpS30 CG15697 3R: 93A2 6e-32 RPLP0 RpLP0 CG7490 3L: 79B2 e-122 RpLP0-like CG1381 2R: 46E5-6 3e-10 RPLP1 RpLP1 CG4087 2L: 21C2 3e-34 RPLP2 RpLP2 CG4918 2R: 53C9 5e-32 RPL3 RpL3 CG4863 3R: 86D8 0.0 RPL4 RpL4 CG5502 3R: 98B6 e-141 RPL5 RpL5 CG17489 2L: h35/40B e-120 RPL6 RpL6 CG11522 3R: 100C7 8e-58 RPL7 RpL7 CG4897 2L: 31B1 2e-75 RpL7-like CG5317 2L: 33C1 3e-32 RPL7A RpL7A CG3314 X: 6B1 e-102 RPL8 RpL8 CG1263 3L: 62E7 e-119 RPL9 RpL9 CG6141 2L: 32C1 1e-66 RPL10 Qm/RpL10 CG17521 3L: h47/80A 7e-98 RPL10A RpL10Aa CG3843 3R: 88D10 3e-65 RpL10Ab CG7283 3L: 68E1 3e-95 RPL11 RpL11 CG7726 2R: 56D7 6e-83 RPL12 RpL12 CG3195 2R: 60B7 7e-75 RPL13 RpL13 CG4651 2L: 30F3 1e-68 RPL13A RpL13A CG1475 3R: 83B6-7 3e-68 RPL14 RpL14 CG6253 3L: 66D8 2e-30 RPL15 RpL15 CG17420 3L: h50-52/80F 5e-90 RPL17 RpL17 CG3203 X: 6C10 5e-72 RPL18 RpL18 CG8615 3L: 65E9 3e-71 RPL18A RpL18A CG6510 2R: 54C3 3e-68 RPL19 RpL19 CG2746 2R: 60E11 4e-83 RPL21 RpL21 CG12775 2L: 40A-B 4e-66 RPL22 RpL22 CG7434 X: 1C4 4e-40 RpL22-like CG9871 2R: 59D3 2e-24 RPL23 RpL23 CG3661 2R: 59B3 1e-68 RPL23A RpL23A CG7977 3L: 62A10 7e-52 RPL24 RpL24 CG9282 2L: 34B10 7e-55 RpL24-like CG6764 3R: 86E5 8e-14 RPL26 RpL26 CG6846 3L: 75E4 1e-59 RPL27 RpL27 CG4759 3R: 96E9-10 1e-43 RPL27A RpL27A CG15442 2L: 24F3 5e-60 RPL28 RpL28 CG12740 3L: 63B14 3e-31 RPL29 RpL29 CG10071 2R: 57D8 8e-14 RPL30 RpL30 CG10652 2L: 37B9 3e-46 RPL31 RpL31 CG1821 2R: 45F5 5e-47 RPL32 RpL32 CG7939 3R: 99D3 6e-58 RPL34 RpL34a CG6090 3R: 96F10 2e-29 RpL34b CG9354 3R: 85D15 1e-29 RPL35 RpL35 CG4111 X: 5A11 2e-38 RPL35A RpL35A CG2099 3R: 83A4 1e-35 RPL36 RpL36 CG7622 X: 1B12 3e-33 RPL36A RpL36A CG7424 2L: 28D3 3e-43 RPL37 RpL37a CG9091 X: 13B1 2e-39 RpL37b CG9873 2R: 59C4 1e-31 RPL37A RpL37A CG5827 2L: 25C4 2e-38 RPL38 RpL38 CG18001 2R: h46/41C-E 2e-25 RPL39 RpL39 CG3997 2R: 60B7 3e-18 RPL40 RpL40 CG2960 2L: 24E1 2e-69 RPL41 RpL41 CG30425 2R: 60E5 8e-08 *Additional gene synonyms exist in most cases [60]. Bold font indicates CRP-like genes, putative pseudogenic fragments (CG11386 and CG33222) or the member of a duplicate gene pair that is expressed in a small number of tissues and/or at relatively low levels. †Computed cytological position is given for euchromatic genes (Genome Release 5 [60]). The cytological and h-band locations for heterochromatic genes are based on data in reference [151] or estimated from images of in situ hybridizations of BACs to polytene chromosomes (RpL5 and RpL21) [152]. The h-band location of RpL15 was provided by B Honda (personal communication). ‡Expect (E) value obtained from a BLASTp search of the D. melanogaster annotated proteome (Genome Release 5.1) with human RefSeq CRP sequences. (E values corresponding to RpL15 and RpS28-like were obtained from a BLAST search using Release 5.3.) Where multiple protein isoforms exist, the highest scoring hit is given. Table 2 The MRP genes of D. melanogaster D. melanogaster gene Human MRP Symbol* CG number Location† BLAST E value‡ MRPS2 mRpS2 CG2937 2L: 25B1 1e-69 MRPS5 mRpS5 CG40049 3L: h47/80A-B 4e-63 MRPS6 mRpS6 CG15016 3L: 64B2 2e-20 MRPS7 mRpS7 CG5108 2L: 31D11 6e-38 MRPS9 mRpS9 CG2957 3R: 84E4 8e-81 MRPS10 mRpS10 CG4247 3R: 88E3 1e-33 MRPS11 mRpS11 CG5184 3R: 89E11 8e-26 MRPS12 tko/mRpS12 CG7925 X: 3A3 1e-33 MRPS14 mRpS14 CG32531 X: 18C7 1e-35 MRPS15 bonsai/mRpS15 CG4207 2R: 58F3 1e-15 MRPS16 mRpS16 CG8338 2R: 50E1 2e-24 MRPS17 mRpS17 CG4326 2R: 60C1 2e-14 MRPS18A mRpS18A CG31450 3R: 85A3 8e-13 MRPS18B mRpS18B CG10757 2L: 38B6 4e-33 MRPS18C mRpS18C CG9688 3R: 99F4 7e-23 MRPS21 mRpS21 CG32854 3R: 87E8 3e-22 MRPS22 mRpS22 CG12261 3R: 98B6 3e-38 MRPS23 mRpS23 CG31842 2L: 34D6 2e-20 MRPS24 mRpS24 CG13608 3R: 95E6 2e-31 MRPS25 mRpS25 CG14413 X: 12F1 1e-49 MRPS26 mRpS26 CG7354 3L: 75B9 3e-11 MRPS27 NA NA NA No hit MRPS28 mRpS28 CG5497 2R: 55E2 1e-27 DAP3/MRPS29 mRpS29 CG3633 2R: 58E1 7e-72 MRPS30 mRpS30 CG8470 X: 13E18 3e-21 MRPS31 mRpS31 CG5904 3L: 72C2 5e-35 MRPS33 mRpS33 CG10406 3R: 89B16 3e-30 MRPS34 mRpS34 CG13037 3L: 72E1-2 8e-06 MRPS35 mRpS35 CG2101 3L: 62F4 2e-72 MRPS36 NA NA NA No hit MRPL1 mRpL1 CG7494 3R: 84F9-10 8e-25 MRPL2 mRpL2 CG7636 3L: 68A7 2e-56 MRPL3 mRpL3 CG8288 X: 13E14 5e-52 MRPL4 mRpL4 CG5818 2L: 35F1 6e-70 MRPL9 mRpL9 CG31478 3R: 88F1 6e-17 MRPL10 mRpL10 CG11488 2L: 21B4 8e-20 MRPL11 mRpL11 CG3351 3R: 88C3 4e-29 MRPL12 mRpL12 CG5012 3L: 66E5 2e-13 MRPL13 mRpL13 CG10603 2L: 37B1 2e-47 MRPL14 mRpL14 CG14048 X: 3A1 3e-31 MRPL15 mRpL15 CG5219 3L: 77C3 4e-74 MRPL16 mRpL16 CG3109 X: 2B14 3e-58 MRPL17 mRpL17 CG13880 3L: 61B3 8e-21 MRPL18 mRpL18 CG12373 2R: 49C2 3e-22 MRPL19 mRpL19 CG8039 3R: 85A5 1e-56 MRPL20 mRpL20 CG11258 3L: 69F5 8e-21 MRPL21 mRpL21 CG9730 3L: 76A3 2e-19 MRPL22 mRpL22 CG4742 X: 15A7-8 4e-41 MRPL23 mRpL23 CG1320 3L: 62D7 4e-28 MRPL24 mRpL24 CG8849 2L: 25B4 2e-45 MRPL27 mRpL27 CG33002 2L: 24F3 2e-10 MRPL28 mRpL28 CG3782 2L: 25B5 5e-27 MRPL30 mRpL30 CG7038 X: 4C11 3e-15 MRPL32 mRpL32 CG12220 3R: 100B8 6e-15 MRPL33 mRpL33 CG3712 X: 4B6 5e-08 MRPL34 mRpL34 CG34147 2R: 52E4 1e-07 MRPL35 mRpL35 CG13410 3R: 94A1 4e-25 MRPL36 mRpL36 CG18767 3L: 66B7 2e-09 MRPL37 mRpL37 CG6547 3R: 86C6 4e-16 MRPL38 mRpL38 CG15871 X: 12E5 2e-61 MRPL39 mRpL39 CG17166 3L: 71B1 2e-57 MRPL40 mRpL40 CG5242 3R: 86E4 6e-13 MRPL41 mRpL41 CG12954 2R: 51E7 2e-11 MRPL42 mRpL42 CG12921 2R: 46E1 1e-11 MRPL43 mRpL43 CG5479 2R: 59F6 1e-25 MRPL44 mRpL44 CG2109 3R: 83A4 6e-39 MRPL45 mRpL45 CG6949 3R: 94B6 4e-61 MRPL46 mRpL46 CG13922 3L: 62B4 5e-39 MRPL47 Rlc1/mRpL47 CG9378 3R: 85D19 1e-35 MRPL48 mRpL48 CG17642 2L: 22B1 4e-15 MRPL49 mRpL49 CG4647 X: 11D1 9e-22 MRPL50 mRpL50 CG8612 3L: 65E9 6e-09 MRPL51 mRpL51 CG13098 2L: 29D4 2e-13 MRPL52 mRpL52 CG1577 2R: 43E9 2e-14 MRPL53 mRpL53 CG30481 2R: 50C16 3e-06 MRPL54 mRpL54 CG9353 2R: 57B16 2e-18 MRPL55 mRpL55 CG14283 3R: 91F1 3e-17 LACTB/MRPL56 NA NA NA No hit *Additional gene synonyms exist in many cases [60]. †Computed cytological position is given for euchromatic genes (Genome Release 5 [60]). mRpS5 h-band location provided by C Smith (DHGP, personal communication) and cytological position inferred from reference [151]. ‡Expect (E) value obtained from a BLASTp search of the D. melanogaster annotated proteome (Genome Release 5.1) with human RefSeq MRP sequences. Where multiple protein isoforms exist, the highest scoring hit is given. Cytoplasmic ribosomal protein genes We identified 88 genes that encode a total of 79 different CRPs (Table 1). Thus, the D. melanogaster proteome contains orthologs of all 79 mammalian CRPs (32 small subunit and 47 large subunit proteins). While the majority of CRPs are encoded by single genes, nine are encoded by two distinct genes. In addition, we identified another five genes predicted to encode proteins with significantly lower similarity to human CRPs, which we term 'CRP-like' genes. Two fragments of the RpS6 gene were also identified. (The list of 88 CRP genes presented by Cherry et al. [64] originated from an earlier report of our results to FlyBase (MA and SJM, FBrf0178764). These authors also list five CRP-like genes from our original report, but two of these have been eliminated and two additional CRP-like genes have been added in the current analysis.) The deduced characteristics of D. melanogaster and human CRPs are compared in Additional data file 1. As might be expected, the amino acid identity between the CRPs of the two species is very high (average of 69% with a range of 27-98%, excluding the CRP-like proteins) and the predicted molecular weights and isoelectric points of the homologous proteins are very similar. However, several D. melanogaster proteins (RpL14, RpL22, RpL23A, RpL29, RpL34a, RpL34b, RpL35A) have significantly lower overall identity and different molecular weights owing to terminal deletions or extensions (data not shown; also see [65]). (If these seven proteins are discounted, the average identity of fly and human CRPs increases to 72% with a range of 43-98%.) Similar to humans and other species, there are very few acidic CRPs in D. melanogaster: only six proteins (RpSA, RpS12, RpS21, RpLP0, RpLP1 and RpLP2) have isoelectric points less than pH 7. (Note that RpS21 is an acidic protein, whereas its human counterpart is basic.) As in other eukaryotes, RpS27A and RpL40 are carboxyl extensions of ubiquitin [66-69], and, as in other animals, RpS30 is fused to a ubiquitin-like sequence. From these gross characterizations of component proteins, it appears that the fly cytoribosome differs only slightly from its human counterpart and is essentially the same as other eukaryotic cytoribosomes. Previous biochemical analyses estimated that the D. melanogaster cytoribosome contains up to 78 CRPs [29]. This figure compares very well to the 79 different CRPs predicted by our orthology analysis (Table 1). Unfortunately, very few of the CRPs identified in the 1970s and 1980s were characterized to the level of amino acid sequence, so their correspondences to CRP genes are generally unknown, though there are a few exceptions (see references [70-73]). We have been unable, therefore, to correlate the CRPs identified in these earlier studies with those encoded by the CRP genes identified in this study. In contrast, our CRP inventory certainly does contain all 52 CRPs identified by the recent biochemical analysis of D. melanogaster cytoribosomes by Alonso and Santarén [34]. Mitochondrial ribosomal protein genes We identified 75 D. melanogaster genes encoding proteins of the mitoribosome (28 in the small subunit and 47 in the large subunit) by orthology to human MRPs (Table 2). These data complement and extend previous analyses of homology between human and D. melanogaster MRPs [16,17]. As in these previous studies, genes encoding orthologs of three human MRPs (MRPS27, MRPS36 and LACTB/MRPL56) were not found. The MRPs of humans and D. melanogaster are much more divergent than are their CRPs: MRPs have an average identity of only 34% (with a range of 15-57%) and several homologous pairs differ markedly in their sizes and isoelectric points (Additional data file 2). Indeed, it is known that the mitoribosome is a rapidly evolving structure whose composition varies among eukaryotic organisms [6]. It is quite possible that there are proteins in Drosophila mitoribosomes that are not found in their human counterparts and these will have been missed by our orthology analysis - a definitive inventory will require biochemical characterization of the fly mitoribosome. As in mammals, three distinct genes encode three different isoforms of MRPS18 (Table 2); it is thought that each mitoribosome contains a single MRPS18 protein and that mitoribosomes may, therefore, be heterogeneous in composition [6]. Duplicate cytoplasmic ribosomal protein genes Of the 79 different CRPs of D. melanogaster, 9 are encoded by two distinct genes (Table 1). These are distinguished by a lowercase 'a' or 'b' suffix to the gene symbol. (The lowercase 'a' should not be confused with the uppercase 'A' suffix used in the standard CRP nomenclature; for example, RpL37a and RpL37A are different genes that encode different proteins.) Six of these gene pairs encode proteins of the small ribosomal subunit and the other three encode large subunit proteins. In humans, each CRP is typically encoded by a single, functional gene [37,74], but thousands of nonfunctional CRP pseudogenes are known to exist [75]. We therefore investigated the evolutionary origin, sequence conservation and expression profile of the duplicate D. melanogaster CRP genes in order to assess whether both members of each pair are likely to be functional (Table 3 and Figure 1). Table 3 Analysis of duplicate CRP genes and CRP-like genes Gene K A /K S b cDNA clones Symbola Location Comments Pair-wisec Branch-specificd Totale % testisf % Amino acid identityg RpS5 RpS5a X: 15E5-7 - 0.07 0.09 133 1 78 RpS5b 3R: 88D6 - 0.09 60 7 RpS6 RpS6 X: 7C2 - NA NA 150 5 33h CG11386 X: 7C2 CG11386 and CG33222 are tandem repeats of the third exon and flanking regions of RpS6 NA 0 0 CG33222 X: 7C2 NA 3 0 RpS10 RpS10a 3R: 98A14 Lacks introns; likely retrogene 0.10 0.15 1 0 73 RpS10b X: 18D3 - 0.01 156 0 RpS14 RpS14a X: 7C6-7 RpS14a and RpS14b share identical gene structures NA NA 154 2 100 RpS14b X: 7C8 NA 12 0 RpS15A RpS15Aa X: 11E11-12 - 0.16 0.00 150 1 98 RpS15Ab 2R: 47C1 Lacks introns; likely retrogene NA 67 1 RpS19 RpS19a X: 14F4 - 0.09 0.01 134 1 65 RpS19b 3R: 95C13 - 0.08 1 100 RpS28 RpS28a 3R: 99D2 Lacks introns; likely retrogene 0.05 0.06 0 0 82 RpS28b X: 8E7 - 0.00 131 1 RpS28-like 2L: 30B3 - NAi NA 1 0 36/37j RpLP0 RpLP0 3L: 79B2 - NA NA 157 2 18 RpLP0-like 2R: 46E5-6 Present in all eukaryotes NA 11 0 RpL7 RpL7 2L: 31B1 - NA NA 168 4 30 RpL7-like 2L: 33C1 - NA 32 0 RpL10A RpL10Aa 3R: 88D10 Lacks introns; likely retrogene 0.05 0.06 7 71 64 RpL10Ab 3L: 68E1 - 0.02 160 4 RpL22 RpL22 X: 1C4 - NA NA 145 2 38 RpL22-like 2R: 59D3 - NA 5 60 RpL24 RpL24 2L: 34B10 - NA NA 162 3 23 RpL24-like 3R: 86E5 Present in all eukaryotes NA 34 3 RpL34 RpL34a 3R: 96F10 RpL34a and RpL34b share identical gene structures 0.04 0.09 56 4 78 RpL34b 3R: 85D15 0.04 132 2 RpL37 RpL37a X: 13B1 - 0.01 NA 158 3 72 RpL37b 2R: 59C4 Lacks introns; likely retrogene 0.04 3 0k aBold font indicates CRP-like genes, putative pseudogenic fragments (CG11386 and CG33222) or the member of a duplicate gene pair that is expressed in a small number of tissues and/or at relatively low levels. b K A /K S calculations are not applicable (NA) to highly diverged sequences or cases where the numbers of both synonymous and nonsynonymous substitutions are very small (<5). cCalculated for each D. melanogaster CRP gene pair using maximum likelihood analysis. Values for pairwise comparisons are shown on the first row of each pair. dBranch-specific score in a three-way maximum likelihood tree including D. pseudoobscura orthologs. A four-way tree was used for RpS19 sequences. eTotal number of cDNA clones (excluding those from cultured cell lines) given in FlyBase [60] (April 2007). RpS28-like cDNA evidence from L Crosby (personal communication). fPercentage of cDNA clones from adult testis cDNA libraries (AT, UT and BS), rounded to the nearest integer. gIdentity between proteins across their whole length. Values for pairwise comparisons are shown on the first row of each pair. hIdentity between RpS6 and the CG11386 or CG33222 protein. If CG11386 or CG33222 were used as alternative third exons of RpS6, the protein encoded would be 60% identical to the conventional RpS6 (see text for details). i RpS28-like is too highly diverged from both RpS28a and RpS28b for a pair-wise K A /K S calculation to be applicable. jIdentity between the RpS28-like protein and RpS28a/RpS28b. kThere is experimental evidence that RpL37b expression is enriched in adult testis [79]. Figure 1 Evolution of D. melanogaster CRP gene duplicates and CRP-like genes. The likely pattern of emergence of CRP duplicate genes with restricted expression (blue), CRP-like genes (green) and CRP pseudogenic fragments (brown) in the lineage leading to D. melanogaster is shown. RpL34b is shown in black text: this is the only case where the newly emerged duplicate gene (RpL34b), rather than precursor gene (RpL34a), acts as the principal gene copy. The relative placement of CG11386 and CG33222 is consistent with the model presented by Stewart and Denell [86]. The dendrogram is based on that given in reference [140], in which the relationships among the Drosophilidae are taken from [149]; note that the branch lengths do not accurately reflect evolutionary time. In five cases, one member of the gene pair lacks introns (RpS10a, RpS15Ab, RpS28a, RpL10Aa and RpL37b) while the other member does not. These five intronless genes are likely to have arisen by retrotransposition; that is, generated via reverse transcription of mRNA from the precursor gene followed by insertion into a new genomic location. In contrast, the RpS5, RpS19 and RpL34 duplicates arose through gene transposition events as both members of each pair retain introns. The RpL34 duplication occurred through an intrachromosomal transposition on chromosome arm 3R, and RpL34a and RpL34b have retained almost identical gene structures. In contrast, the RpS5 and RpS19 duplications involved interchromosomal transposition events that must have been followed by extensive gene remodeling as the intron-exon structures differ within each pair. Finally, RpS14a and RpS14b probably arose via unequal exchange: these paralogs are situated adjacent to each other as a tandem duplication on the X chromosome, share identical intron-exon structures and encode identical proteins [76]. All nine duplicate genes appear to have arisen within the Drosophilidae, albeit at different stages in the lineage leading to D. melanogaster (Figure 1). Neither member of these 9 CRP gene pairs contains a nonsense mutation in the protein-coding region (data not shown), indicating that all 18 genes are potentially functional. Moreover, the low ratio of nonsynonymous to synonymous substitutions (K A /K S ) between the members of each gene pair suggests that there are selective constraints on their protein-coding regions (Table 3; a K A /K S ratio significantly lower than 0.5 indicates functional constraints on both genes). Branch-specific K A /K S values further indicate that the putatively retrotransposed genes have been under overall purifying selection since their formation. Together, these data argue that none of these duplicate genes are nonfunctional pseudogenes, which is consistent with a previous analysis [77]. Indeed, the recovery of multiple cDNA clones for the majority (15/18) of these duplicate genes supports their expression in vivo (Table 3). Although none of these CRP gene duplicates appear to be pseudogenes, it is evident that one member of each pair - the one with higher similarity to its human ortholog, where this difference exists (Table 1 and Additional data file 1) - is expressed at a significantly higher level and, in some cases, in a wider array of tissues than the other. This suggests that one gene of the pair produces the majority of each CRP in most cells, while the other gene has a more restricted expression pattern and, perhaps, a specialized function (indicated by bold font in Tables 1 and 3). In eight of the nine duplication events, the 'younger' gene copy has adopted the lower expression level or more restricted expression pattern; the RpL34 gene pair is exceptional in this regard (Figure 1 and Table 3). The expression of RpS5b, RpS19b, RpL10Aa and RpL37b appears enriched in the adult testis, suggesting the existence of testis-specific CRPs and a testis-specific cytoribosome (Table 3). Significantly, three of these genes (RpS5b, RpS19b and RpL37b), together with RpS10a, RpS15Ab and RpS28a, are autosomal copies of X-linked genes. These duplication events are consistent with previous studies reporting that genes with male-biased expression are predominantly autosomal [78], and that retrotransposed genes in D. melanogaster have preferentially retrotransposed from the X chromosome onto autosomes [79]. It is possible that these autosomal duplicates enable CRP expression in male germline cells, where it is hypothesized that X chromosome inactivation occurs during spermatogenesis [80]. Similarly, in humans, RPS4Y is a Y-linked duplicate of the X-linked RPS4 gene [10] and RPL10L, RPL36AL and RPL39L are autosomal retrogene copies of X-linked progenitors [74]. It is worth noting that expression of D. melanogaster RpS5b, RpS10a and RpS19b is also enriched in the germline cells of embryonic gonads [81] and/or stem cells of adult ovaries [82]. These findings suggest a germline-specific role, rather than a testis-specific role, for these CRP gene duplicates. To conclude, the 'principal' CRPs of D. melanogaster - those that are expressed at high levels in most cells - are each encoded by single genes. Cytoplasmic ribosomal protein-like genes We identified five D. melanogaster 'CRP-like' genes that encode proteins with significantly lower identity to human CRPs than those described above. These are RpS28-like, RpLP0-like, RpL7-like, RpL22-like and RpL24-like (shown in bold font in Tables 1 and 3). Of these, RpLP0-like and RpL24-like show the most divergence from their cognate proteins, RpLP0 and RpL24. Consistent with this, RpLP0-like and RpL24-like have ancient evolutionary origins, while RpL7-like, RpL22-like and RpS28-like arose more recently within the Diptera (Figure 1). cDNA evidence indicates that all five of these CRP-like genes are expressed in vivo, albeit at far lower levels than their cognate genes (Table 3). The evolutionary conservation of RpLP0-like and RpL24-like suggests they have important cellular functions. Indeed, the yeast ortholog of RpL24-like is found in pre-ribosomal complexes where it is thought to function in large subunit biogenesis [83]. It remains to be seen whether the other CRP-like proteins have similar functions. Interestingly, the RpL22 gene is X-linked and expressed ubiquitously, whereas RpL22-like is an autosomal gene that is expressed predominantly in germline cells [81,82,84,85]. This suggests that RpL22-like may have a specialized role in the germline, and perhaps within germline-specific cytoribosomes, as proposed above for some of the CRP duplicates. CG11386 and CG33222 are 99% identical in DNA sequence and are tandem repeats of the third exon and flanking regions of the RpS6 gene. They likely arose via two sequential unequal crossover events [86]; the first occurring after the evolutionary split of the melanogaster subgroup, and the second being specific to D. melanogaster (Figure 1). Gene prediction algorithms suggest that CG11386 and CG33222 are distinct genes encoding identical amino-terminally truncated versions of RpS6 [87]; however, such proteins would lack critical functional domains and would probably be nonfunctional. In a different scenario, CG11386 and/or CG33222 could serve as alternative third exons of the RpS6 gene: the proteins produced would be full-length, but would differ substantially in their carboxy-terminal two-thirds from the RpS6 generated by using the conventional third exon [86]. There is, however, no direct evidence that such alternative transcripts are made. Indeed, only three cDNA clones suggest that CG11386 or CG33222 are expressed at all (Table 3). We have tentatively classified CG11386 and CG33222 as nonfunctional pseudogenic fragments. Chromosomal distribution of ribosomal protein genes As has been found for other eukaryotes [35,36,38,39], the RP genes of D. melanogaster are distributed across the entire genome (Figure 2). Some RP genes are tightly linked to other RP genes and, while this posed challenges for determining the phenotypes associated with individual genes (see below), we have no evidence that this distribution has functional consequences or that closely linked RP genes are transcriptionally co-regulated. Five RP genes (RpL5, Qm/RpL10, RpL15, RpL38, and mRpS5) are located within heterochromatic regions, as are certain human MRP genes [38] and some Arabidopsis thaliana CRP genes [36]. As heterochromatin is generally associated with the silencing of gene expression [88], the regulation of these genes must have adapted to the heterochromatic environment in order for the encoded proteins to be expressed at sufficiently high levels to meet the demand for ribosome synthesis in the cell [89]. Figure 2 Chromosomal map of the RP genes of D. melanogaster. RP genes are depicted on a physical map of the genome (Release 5) [60]. Genes encoded on the positive and negative strands are shown above and below the chromosome, respectively. (The orientation of RpL15 is not known and its position below the chromosome is arbitrary.) Chromosomes are divided into cytological bands as determined from sequence-to-cytogenetic band correspondence tables [150]. Minute genes are boxed as described in the key. Ribosomal protein gene haploinsufficiency and the Minute syndrome Classical genetic studies have defined more than fifty regions of the D. melanogaster genome that are haploinsufficient and associated with the dominant phenotypes of prolonged development and short, thin bristles - the Minute loci [2] (Figure 3). To date, only fifteen Minute loci have been tied unequivocally to molecularly defined genes and all of these encode RPs (reviewed in reference [2]; also see references [48-53]). It has not been clear, however, if all Minute loci correspond to RP genes, or whether Minute loci may correspond to both CRP and MRP genes. We have conducted a new survey of Minute loci in the D. melanogaster genome which, combined with our RP gene inventory, has now allowed us to assess these relationships systematically. Figure 3 The Minute bristle phenotype. Minute flies have shorter and thinner bristles than wild type flies. This is most clearly seen by comparing the scutellar bristles, indicated here by the arrows and pseudocoloring. (a, a') Wild type. (b, b') RpS13 1 heterozygotes. (c, c') RpL14 1 heterozygotes. Recent large-scale projects have provided a wealth of new genetic reagents that enable the mapping of Minute loci with a precision unavailable only a few years ago. Hundreds of new deletions with molecularly defined breakpoints have been provided by the efforts of the DrosDel consortium [90,91], Exelixis, Inc. [92], and the Bloomington Drosophila Stock Center [92]. When combined with older deletions characterized primarily through polytene chromosome cytology, these deletions have increased euchromatic genome coverage to 96-97%. In addition, transposable element insertions now exist within 0.5 kb of 57% of all genes (R Levis, personal communication), largely through the efforts of the Drosophila Gene Disruption Project [93] and Exelixis, Inc. [94]. We used these resources to conduct a genome-wide search for Minute loci. In so doing, we considered the characteristic Minute bristle phenotype (Figure 3) to be diagnostic of the Minute syndrome; we did not methodically evaluate more subtle Minute traits, such as slower development, or traits observed in only a subset of Minute mutants, such as impaired fecundity, reduced viability or altered body size. By combining our observations with information gleaned from published studies, we have identified 61 distinct Minute loci. Many of these correlate with Minute loci described previously (Additional data file 3), though our work has often refined their map positions. Significantly, six Minute loci (M(2)31E, M(2)34BC, M(2)45F, M(2)50E, M(3)93A and M(3)98B) are reported here for the first time. We also found four instances (M(2)31A, M(2)53, M(2)58F and M(3)67C) where a single Minute locus characterized by previous aneuploidy analyses actually comprises two separable, closely linked Minute genes. As we have inferred the existence of four additional Minute loci from patterns of deletion coverage (described below), we conclude that there are 65 distinct Minute loci in the D. melanogaster genome. We were able to demonstrate definitively that a particular Minute locus corresponds to a specific RP gene when a Minute bristle phenotype was observed in one or more of the following situations: flies heterozygous for a molecularly characterized mutation in a RP gene (for example, M(2)36F/RpS26); flies heterozygous for a chromosomal deletion when the Minute phenotype could be mapped unambiguously to a single RP gene with deletion breakpoints (for example, M(2)25C/RpL37A); or flies heterozygous for a chromosomal deletion when the Minute phenotype could be rescued by a specific RP transgene (for example, M(3)99D/RpL32). We found that there are 26 unequivocally Minute CRP genes by these criteria (Additional data file 4; summarized in Table 4). In contrast, no MRP or CRP-like genes were definitively demonstrated to be Minute genes. Table 4 CRP gene haploinsufficiency CRP genea Genetic analysisb Symbol Location CRP gene mutationsc, d Deletions removing a single CRP genec, e Other evidence Is the CRP gene a Minute?f No. of candidate Minute genesg Minute synonymh Referencei X chromosome RpL36 1B12 M Yes M(1)1B [53] RpL22 1C4 + + No sta/RpSA 2B1 + + No RpL35 5A11 Lies in a gap in deletion coverage. 5A6-13 aneuploids were Minute (Merriam et al. [55]) Likely 33 M(1)5A RpL7A 6B1 M Likely 2 M(1)5D6A RpL17 6C10 Lies in a gap in deletion coverage Likely 12 New? RpS6 7C2 M M Yes M(1)7BC and M(1)7C [2] RpS14a 7C6-7 A deletion that removes RpS14a and RpS14b is not Minute [95] No RpS14b 7C8 A deletion that removes RpS14a and RpS14b is not Minute [95] No RpS28b 8E7 Lies in a gap in deletion coverage. A Minute mutation was mapped to 8D8-9A2 [153] Likely 17 M(1)8F RpS15Aa 11E11-12 M Likely 8 M(1)11F RpL37a 13B1 Lies in a gap in deletion coverage. 12F6-13B6 aneuploids were Minute (Merriam et al. [55]) Likely 2 M(1)13A RpS19a 14F4 M Likely 14 M(1)14E RpS5a 15E5-7 M Lies in a gap in deletion coverage Yes M(1)15D [44] RpS10b 18D3 M M Yes M(1)18C Chromosome arm 2L RpLP1 21C2 M M Yes M(2)21C [50] oho23B/RpS21 23B6 M M Yes M(2)23B [49] RpL40 24E1 M Likely 4 M(2)24D RpL27A 24F3 M M Yes M(2)24F RpL37A 25C4 M The interval between flanking non-Minute deletions contains only RpL37A Yes M(2)25C RpL36A 28D3 M Likely 5 M(2)28DE* RpS13 29B2 M M Yes M(2)29B* [45] sop/RpS2 30E1 + + No RpL13 30F3 M The interval between flanking non-Minute deletions contains only RpL13 Yes M(2)31A RpL7 31B1 M Likely 2 M(2)31A RpS27A 31E1 M Likely 6 M(2)31E* RpL9 32C1 M M Yes M(2)32D [46] RpL24 34B10 M Likely 8 M(2)34BC* RpS26 36F4 M M Yes M(2)36F RpL30 37B9 + + No RpL21 40A-B M Likely 10 M(2)39F RpL5 40B M M Yes M(2)40B* [51] Chromosome arm 2R RpL38 41C-E M M Yes M(2)41A [51] RpL31 45F5 M Lies in a gap in deletion coverage Yes M(2)45F* RpS15Ab 47C1 + No RpS11 48E8-9 M Lies in a gap in deletion coverage Yes M(2)48E* RpS23 50E4 M Likely 2 M(2)50E* RpS15 53C8 M(2)53 1 Minute phenotype rescued by duplication of 51F-54A [154] but not by a RpLP2 transgene [155] Likely 16 M(2)53 RpLP2 53C9 M Likely 5 M(2)53 RpL18A 54C3 Lies in a gap in deletion coverage Likely 16 New? RpL11 56D7 Lies in a gap in deletion coverage. 56C-D aneuploids were Minute [55] Likely 3 M(2)56CD RpS18 56F11 M Likely 2 M(2)56F RpL29 57D8 + No RpS16 58F1 Deletions that remove both RpS16 and RpS24 are Minute. The Minute mutations M(2)58F 1 and RpS24 SH2053 complement, suggesting RpS16 is a Minute gene Likely 25 M(2)58F RpS24 58F3 M Deletions that remove both RpS16 and RpS24 are Minute Yes M(2)58F RpL23 59B3 + No RpL37b 59C4 + No RpL12 60B7 RpL12 and RpL39 lie in the same gap in deletion coverage. 60B3-10 aneuploids were Minute [156] Likely 9 M(2)60B RpL39 60B7 RpL12 and RpL39 lie in the same gap in deletion coverage. 60B3-10 aneuploids were Minute [156] Likely 9 M(2)60B RpL41 60E5 + + No RpL19 60E11 M A deletion that removes RpL19 and RpL41 is Minute Yes M(2)60E [42] Chromosome arm 3L RpL23A 62A10 M Likely 8 M(3)62A RpL8 62E7 Lies in a gap in deletion coverage containing only RpL8. 62E-63A aneuploids were Minute [54] Yes M(3)62F RpL28 63B14 M Likely 10 M(3)63B RpL18 65E9 M Likely 8 M(3)65F RpL14 66D8 M Lies in a gap in deletion coverage Yes M(3)66D [47] RpS17 67B5 A deletion removing both RpS17 and RpS9 is Minute. The unsequenced Minute mutations RpS17 4 and RpS17 6 complement the Minute mutation RpS9 EP3299, suggesting RpS17 is a Minute gene Likely 13 M(3)67C RpS9 67B11 M A deletion removing both RpS17 and RpS9 is Minute Yes M(3)67C RpL10Ab 68E1 + No RpS12 69F5 + A deletion that removes RpS12 and RpS4 is Minute No RpS4 69F6 A deletion that removes RpS12 and RpS4 is Minute Likely 2 M(3)69E RpL26 75E4 + No RpLP0 79B2 + + No Qm/RpL10 80A M Likely 23 M(3)80 RpL15 80F M Likely = 11 M(3)80F* Chromosome arm 3R RpL35A 83A4 Lies in a gap in deletion coverage Likely 3 New? RpL13A 83B6-7 M Lies in a gap in deletion coverage Yes M(3)83B* [52] RpL34b 85D15 Lies in a gap in deletion coverage Likely 3 New? RpS29 85E8 M M Yes M(3)85E RpS25 86D8 A deletion that removes RpS25 and RpL3 is Minute Likely 14 M(3)86D RpL3 86D8 + No RpS5b 88D6 + + No RpL10Aa 88D10 + No RpS20 93A1 A deletion that removes RpS20 and RpS30 is Minute Likely 21 M(3)93A* RpS30 93A2 A deletion that removes RpS20 and RpS30 is Minute Likely 21 M(3)93A* RpS3 94E13 M M Yes M(3)95A [43] RpS19b 95C13 + No RpS27 96C8 M Likely 5 M(3)96C RpL27 96E9-10 M Likely 6 M(3)96CF RpL34a 96F10 + No RpS10a 98A14 + No RpL4 98B6 M Likely 6 M(3)98B* RpS8 99C4 Lies in a gap in deletion coverage. 99B aneuploids were Minute [157] Likely 11 M(3)99B RpS28a 99D2 The Minute phenotype of a deletion removing RpS28a and RpL32 is rescued by a RpL32 transgene [41] No RpL32 99D3 The Minute phenotype of a deletion removing RpS28a and RpL32 is rescued by a RpL32 transgene [41] Yes M(3)99D [41] RpS7 99E2 Lies in a gap in deletion coverage. 99E-F aneuploids were Minute [157] Likely 11 M(3)99E RpL6 100C7 Lies in a gap in deletion coverage. 100C-F aneuploids were Minute [157] Likely 16 M(3)100CF Chromosome 4 RpS3A 101F1 M M Yes M(4)101 [48] aBold font indicates the member of a duplicate gene pair that is expressed in a small number of tissues and/or at relatively low levels. bComplete details are given in Additional data file 4. c'M' indicates that mutation or deletion heterozygotes display a Minute bristle phenotype; '+' indicates they are wild type; a blank indicates the absence of appropriate mutations or deletions. dMutations mapped molecularly to a single CRP gene. eDeletions removing several genes, but only a single CRP gene. fJudged according to evidence summarized in previous three columns and presented in detail in Additional data file 4. gThe maximum number of genes that could correspond to the Minute; defined as the number of genes between the relevant deletion breakpoints minus the number of genes with non-Minute mutations. h Minute synonyms from literature sources (see Additional data files 3 and 4). Asterisks indicate new synonyms assigned in this study. iReference demonstrating definite correspondence between a Minute locus and CRP gene. Where no reference is given, the correspondence is shown for the first time in this study. These 26 cases of proven CRP gene-Minute locus correspondences provide a strong precedent for expecting that other CRP genes are also Minute genes. Although existing reagents do not allow us to demonstrate the correspondences definitively, we judged that a CRP gene very likely corresponds to a genetically defined Minute locus when one or more of the following criteria are fulfilled: a Minute phenotype is seen for a heterozygous multi-gene deletion that uncovers a single CRP gene (for example, M(3)63B/RpL28); a CRP gene lies in a gap in deletion coverage and a molecularly uncharacterized Minute mutation maps to the same region (for example, M(1)8F/RpS28b); or a CRP gene lies in a gap in deletion coverage and previous studies of transient aneuploids document the presence of a Minute locus in the same region (for example, M(3)99E/RpS7). In this way, we identified an additional 36 CRP genes that likely correspond to 34 genetically defined Minute loci (Additional data file 4; summarized in Table 4). Closely linked pairs of CRP genes map to the same regions as M(2)60B and M(3)93A and, as it was impossible to determine whether one or both genes of each pair are haploinsufficient, we have classified all four CRP genes as likely Minute genes. No CRP-like genes mapped to the regions of proven Minute loci. Although five MRP genes map to regions containing Minute loci, it is unlikely that any of them are haploinsufficient: MRP genes are not associated with Minute phenotypes in any other situation, and each of these five MRP genes is closely linked to a CRP gene (Additional data file 4). We concluded that a further four CRP genes (RpL17, RpL18A, RpL34b and RpL35A) are likely to be Minute genes despite no Minute phenotype having been associated with the genomic region in which they reside. In each of these cases, the CRP gene lies in a gap in deletion coverage (Table 4, Additional data file 4), suggesting that it is a Minute associated with strongly reduced fertility and/or viability, which prevents the establishment of stable deletion stocks (in the absence of a corresponding duplication). Supporting this view, such severe haploinsufficiency also appears to be associated with 15 other CRP genes - all these CRP genes lie in gaps in deletion coverage and they are only considered Minute genes here because they have point or transposon insertion (likely hypomorphic) mutations that cause Minute phenotypes, or because they lie in regions known to harbour Minute loci from the phenotypes of transient aneuploids (Table 4, Additional data file 4). For all of the 40 CRP genes classified as 'likely Minute genes' (through correlation with genetically proven Minute loci or gaps in deletion coverage), we determined the maximum number of candidate genes that could possibly account for the haploinsufficiency. We used deletions to define the smallest chromosomal interval containing the Minute and then eliminated genes known not to be associated with a Minute phenotype from previous studies or from our own examinations of mutant fly strains. (This task benefited greatly from the recent work of the Bloomington Drosophila Stock Center which, in its efforts to maximize genomic deletion coverage, has systematically generated deletions flanking haploinsufficient loci.) The number of candidate genes defined in this way was always small, ranging from 2 to 33 genes with a median of 8.5 candidate genes per Minute locus (Table 4, Additional data file 4). These data increase our confidence in the likely correspondences between these Minute loci and CRP genes. The results presented above indicate that 66 CRP genes are, or are likely to be, Minute genes, whereas the remaining 22 CRP genes are not (Table 4 and Additional data files 4 and 5; summarized in Figure 4). CRPs of the large and small ribosomal subunit are encoded by both Minute and non-Minute genes, with no apparent bias. Notably, none of the nine duplicate CRP genes with relatively restricted expression is a Minute, whereas seven of the more highly and widely expressed gene pair members are Minute genes. This is consistent with the idea that only one member of each of these gene pairs contributes significantly to cytoribosomal function in the majority of cells, while the one with restricted expression encodes a component of qualitatively distinct cytoribosomes in certain cell types. As the 'principal' copy of RpS14 or RpL10A is not a Minute, it is unsurprising that the simultaneous heterozygous deletion of both RpS14 genes [95] or both RpL10A genes (in flies with genotypes Df(3L)ED4475/Df(3R)ED10556 or Df(3L)ED4475/Df(3R)ED5660; our observations) does not produce flies with Minute phenotypes. Other possible reasons for different dosage sensitivities among CRP genes are discussed below. Figure 4 Summary of Minute locus - CRP gene correspondences. This figure shows the relationship between Minute loci defined by genetic criteria and CRP genes identified using bioinformatics. '=' indicates definite correspondence, '~' indicates probable correspondence. Daggers mark Minute loci that we know or strongly suspect correspond to two CRP genes (as detailed in Table 4 and Additional data file 4). The one verified Minute locus that does not correspond to a CRP gene is M(1)14C. We mapped this Minute gene to region 14C6 by showing that the deletions Df(1)ED7364 (14A8;14C6) and Df(1)FDD-0230908 (14C6;14E1) are each associated with a Minute phenotype. Moreover, we could rescue these phenotypes, as well as the Minute phenotype associated with the M(1)14C 815-29 point mutation [44], using the small tandem duplication Dp(1;1)FDDP-0024486 (14C4;14D1). The Minute region defined by these experiments contains only two genes: CG4420 and eIF-2α. Significantly, flies heterozygous for P{RS5}eIF-2α 5-HA-1790, an insertion in the 5' untranslated region (UTR) of eIF-2α that creates a likely hypomorphic allele, show a discernable, albeit weak Minute phenotype (our observations). This identifies eIF-2α as M(1)14C. Consistent with this conclusion, flies expressing a dominant-negative eIF-2α protein grow slowly and attain a small body size [96], phenotypes that are typical of the Minute syndrome. eIF-2α is one of the three subunits that constitute eIF2, a key translation initiation factor that delivers the methionine-loaded initiator tRNA to the ribosome by transiently associating with the small cytoribosomal subunit [97]. Although eIF-2α is not a component of cytoribosome complexes isolated by standard biochemical preparations, a reduction in eIF-2α gene dosage might still be expected to adversely affect cytoribosomal function and decrease overall rates of protein synthesis by specifically impairing translation initiation. Interestingly, the gene encoding eIF-2γ, another subunit of the eIF2 translation initiation factor, is also haploinsufficient. Transcripts from the Su(var)3-9 gene are alternatively spliced to produce two different proteins with distinct functions: one protein is the eIF-2γ translation factor, the other is responsible for suppression of position effect variegation [98]. Mutations that specifically eliminate the suppressor protein are homozygous viable and are not associated with Minute phenotypes [99], but deletions of the entire gene are haplolethal in the absence of P{(ry +), 11. 5kb}, a transgenic construct carrying the complete Su(var)3-9 genomic region (our observations; Additional data file 6). These data indicate that the regions of the Su(var)3-9 transcription unit encoding eIF-2γ are haplolethal. Moreover, it is possible that this haplolethality actually represents an extreme Minute phenotype associated with the eIF-2γ-coding regions; hypomorphic eIF-2γ mutants, if isolated, may show less severe Minute phenotypes. To assess the possibility that other translation factor genes might also be haploinsufficient/Minute, we examined the heterozygous loss-of-function phenotypes of 68 translation factor genes we identified from BLAST searches and/or Gene Ontology classification (Additional data file 6). We identified no other cases of haploinsufficiency, though five genes could not be assessed with existing deletion and mutation strains. In contrast to the genes encoding the other two subunits of eIF2, the eIF-2β gene is not haploinsufficient. As mentioned above, we have compared our inventory of Minute genes with the Minute loci defined and named from previous genetic analyses (Additional data file 3). In so doing, we failed to validate the existence of several Minute loci described in the past, namely M(1)3E [55], M(1)4BC [55], M(2)21AB [100-102], M(2)44C [55], M(3)76A [55], M(3)82BC [55] and M(3)96A [103]. The existence of some of these loci has been questioned previously and many cases appear to have involved chromosomal aberrations that were unusually complex or point mutations that were mismapped. Our failure to observe a Minute phenotype for deletions of S-adenosylmethionine synthetase (Sam-S), also known as M(2)21AB, is consistent with the phenotypic instability of dominant Sam-S mutations documented previously [100-102]. This suggests that mutations in Sam-S can phenocopy Minute mutations under certain conditions, but that Sam-S is not a typical Minute gene. In summary, CRP genes are likely to correspond to all but one of the 65 Minute loci defined in this study, with the sole exception encoding a translation initiation factor subunit (Figure 4). No MRP or CRP-like genes are unequivocally associated with a Minute phenotype, indicating that the Minute syndrome is specifically related to the function of the cytoribosomes responsible for the majority of cellular protein translation, rather than the function of specialized cytoribosomal variants. Twenty-five percent of CRP genes are not associated with an obvious haploinsufficient phenotype, clearly reinforcing previous findings that not all CRP genes are Minute genes [61,95,104]. Discussion CRP gene haploinsufficiency and the cytoribosome When one examines the phenotypes of flies carrying chromosomal deletions, one is struck by the remarkable tolerance of Drosophila to aneuploidy: flies heterozygous for deletions of hundreds of kilobases of DNA usually have no obvious dominant phenotypes. For this reason, the haploinsufficiency of single genes is all the more remarkable. It is even more striking that the vast majority of these haploinsufficient genes encode proteins of the cytoribosome and that haploinsufficiency is not apparent for genes encoding components of equally elaborate cellular complexes, such as mitoribosomes or spliceosomes. What accounts for the exquisite dosage sensitivity of CRP genes? The primary cause of CRP gene haploinsufficiency is reasonably clear: halving the copy number of a CRP gene results in reduced mRNA expression of that CRP [105]. Similarly, depleting CRP mRNAs through antisense- or RNA interference (RNAi)-mediated approaches can also produce Minute phenotypes [106,107] (SJM and SJL, unpublished data). As there appear to be no compensatory increases in transcription [105], reducing dosages of CRP genes must result in reduced CRP protein levels in the absence of dramatic changes in mRNA stability or CRP protein turnover. How then does the reduction in the level of a single CRP result in impaired cytoribosomal function and reduced general protein synthesis, and how is this manifested as the Minute phenotype? One possibility, termed the 'balance hypothesis' [108,109], is linked to the multisubunit nature of the cytoribosome. It posits that an imbalance in the concentrations of CRPs results in the assembly of incomplete and non-functional ribosomal subunits. Indeed, it is known that depletion of individual CRPs in Saccharomyces cerevisiae causes inefficient ribosomal subunit assembly and/or function [110,111]. Nevertheless, the balance hypothesis also predicts that overexpression of individual CRPs should cause stoichiometric imbalances and phenotypes similar to those produced by underexpression. This prediction is not upheld in either S. cerevisiae [112] or D. melanogaster [113]. Consequently, imbalance per se cannot account for the haploinsufficiency of CRP genes. A simpler explanation of CRP haploinsufficiency is that a high concentration of cytoribosomes is required for proper cellular functions and that the cytoribosome population decreases sharply when the level of a single CRP is reduced. Cytoribosomes and their components do appear to be required in unusually high quantities: CRP mRNAs are among the most abundant cellular transcripts both in yeast [114] and in flies [60], and can account for 50% of all RNA polymerase II-mediated transcription [89]. What seems critical, however, to the high-level production of fully formed cytoribosomes is that the concentration of each and every CRP never falls below a minimal level. In other words, cytoribosomal assembly is strictly limited by the availability of the least abundant component. This is probably not just a matter of simple self-assembly kinetics as improperly assembled ribosomal subunits and excess CRPs are actively degraded in yeast [111,115]. Similarly, RNAi-mediated depletion of single CRPs leads to the depletion of other CRPs in flies [64], suggesting the existence of similar degradation processes. Consequently, halving the supply of a single, limiting CRP is expected to halve the number of functional cytoribosomes. This may be tolerated by many cellular processes but will have severe effects wherever high protein synthesis is required, such as bristle formation and oocyte production in flies, or growth of S. cerevisiae in rich media [116]. It appears, therefore, that it is the combination of high demand for cytoribosomes and an assembly mechanism that assures that the level of the least abundant CRP determines the final concentration of cytoribosomes that makes CRP genes so exquisitely and specifically dosage sensitive. This perspective also provides a context for understanding the non-additivity of Minute mutations [117], where combinations of Minute mutations usually do not have a cumulative effect, but rather result in a phenotype similar to that of the most severe individual Minute mutant. If adequate levels of cytoribosomes depend not so much on precise equimolar CRP concentrations as a minimal concentration of each and every CRP, then we should expect that variation in the expression of different CRPs (above the minimum level) might normally be tolerated in vivo. Such variation could be the result of differences in rates of gene transcription, mRNA translation, or mRNA/protein stability. This view provides a framework for understanding the spectrum of haploinsufficient phenotypes associated with CRP genes, which ranges from no obvious phenotypes, through bristle defects and reduced fecundity and viability, to dominant sterility or haplolethality in the most severe cases. That is, the severity of Minute phenotypes may be related to the rates at which individual CRPs are normally produced [105,106]. In reality, the explanation of CRP gene haploinsufficiency is probably more complicated than cytoribosome assembly relying simply on minimal CRP concentrations. The exact function, position or stoichiometry of CRPs within the cytoribosome may determine whether its gene is haploinsufficient and the severity of the Minute phenotype. For instance, our finding that the gene encoding the eIF-2α translation initiation factor subunit is a Minute could indicate that haploinsufficient CRP genes encode ribosomal components involved specifically in translation initiation. As another example, RpLP1 and RpLP2 are the only CRPs required in two copies per cytoribosome [15] and, consequently, they must be produced at twice the level of all other CRPs. It is perhaps not surprising, therefore, that both RpLP1 and RpLP2 are haploinsufficient (Table 4) [50]. It may even be the case that the haploinsufficiency of some CRP genes arises by less conventional mechanisms. For example, the introns of 27 CRP genes host genes for small nucleolar RNAs (snoRNAs) [118-122], a class of non-coding RNAs that guide post-transcriptional modifications of rRNA molecules necessary for the maturation and incorporation of rRNA into ribosomes [123]. The expression of intronic snoRNAs depends upon the expression and processing of mRNAs from the host gene [124]. Consequently, mutations that reduce expression or splicing of CRP transcripts harboring snoRNAs will simultaneously deplete the cell of both a CRP and properly processed rRNA molecules, thereby impairing cytoribosome biogenesis in two different ways. Although 21 of the 27 CRP genes that carry snoRNA genes within their introns are Minute or likely Minute genes (data not shown), the presence of intronic snoRNA genes cannot be the sole factor determining CRP gene haploinsufficiency. Indeed, we have not found any single factor that clearly determines the degree of dosage sensitivity exhibited by different CRP genes. Regardless of the exact causes and mechanisms of haploinsufficiency, it is pertinent to ask why the majority of CRPs are expressed so close to the level of sufficiency, such that loss of one gene copy is debilitating, rather than being synthesized in excess? One possibility concerns economics: cytoribosomal synthesis is an incredibly costly affair [89] and excessive CRP production would both be wasteful and monopolize the limited resources of the cell. A second possibility is that CRP levels are normally constrained to guard against inappropriate activation of cell growth, proliferation or apoptosis - processes in which CRPs and cytoribosomes have been postulated to play direct roles [125]. A final and intriguing possibility is that the barely sufficient expression levels of some CRP genes may have evolved as a viral defense mechanism. Cherry et al. [64] found that reducing the levels of 64 of the 79 principal CRPs by RNAi inhibits the propagation of Drosophila C virus in Drosophila adults and cultured cells. Because this virus requires high concentrations of cytoribosomes in its host cell to undergo efficient translation, tightly controlled expression of CRP genes at levels just sufficient for normal growth and development may protect against viral infection and provide a selective advantage. Interestingly, we found a modest correlation between a CRP gene being a Minute and it being able to inhibit virus replication in this assay. Clearly, further work will be required to test whether there is truly a relationship between normal CRP gene expression levels and susceptibility to viruses. Minute mutations attracted the attention of early geneticists because they were isolated so often in D. melanogaster. In fact, Schultz said in 1929, "...so many have been found that this mutant type is one of the most frequent in Drosophila" [117]. As a considerable number of Minute mutants have also been isolated in other Drosophila species [60], one might be justified in thinking Drosophila are unusually sensitive to CRP gene haploinsufficiency. On the other hand, the phenotypic consequences of CRP gene haploinsufficiency may simply be more noticeable in flies because they include conspicuous changes in external morphology. In fact, 'Minute-ness' may be a widespread phenomenon that is under-recognized because CRP gene haploinsufficiency has different and varied phenotypic consequences in other organisms. Recent research suggests this is the case [116,126-132]. For example, RPS5 haploinsufficiency disrupts cell division and causes developmental and growth phenotypes in Arabidopsis [126]; several CRP genes are haploinsufficient for suppression of nerve sheath tumors in zebrafish [127]; and RPS19 haploinsufficiency is a causative factor of Diamond-Blackfan anemia in humans [128,130]. In fact, our reliance on the obvious bristle phenotype to distinguish Minute from non-Minute loci may present a biased assessment of CRP gene haploinsufficiency in the fly: it is quite possible that the 22 CRP genes classified as non-Minute in this study are associated with more subtle haploinsufficient phenotypes. How reduced CRP expression gives rise to diverse phenotypes is a mystery that, at least in part, reflects our current ignorance of the regulation and roles of CRPs in different cell types. This is certainly a topic worthy of more research. Conclusion We have assessed an idea that has been discussed for more than three decades; namely, that the haploinsufficient Minute loci of Drosophila correspond to the genes encoding protein components of ribosomes [2,133]. Our results confirm this idea and add important details. We have shown that Minute genes encode proteins of cytoplasmic ribosomes and not mitochondrial ribosomes, and we have defined the subset of CRP genes that are haploinsufficient. While duplicate genes encoding tissue-specific CRPs are not associated with Minute phenotypes, it is not otherwise clear what distinguishes the CRP genes that are haploinsufficient from those that are not. We identified a single Minute gene encoding a different kind of protein, a cytoplasmic translation initiation factor subunit. This hints that haploinsufficient CRP genes may encode proteins specifically involved in translation initiation, although further work is obviously needed to test this idea. Minute genes account for the vast majority of the haploinsufficient genes in the D. melanogaster genome with effects on fertility and viability strong enough to prevent the recovery of chromosomal deletions in the absence of corresponding duplications. Indeed, there are very few additional genes (for example, dpp [134], Abd-B [135]) or chromosomal regions (for example, Tpl [136], wupA [137], Fs(1)10A [138]) unequivocally associated with haplolethality or haplosterility. (A few other regions have been reported but not investigated in detail.) The most immediate practical use for our data will be in systematic efforts to maximize genome deletion coverage. Knowing which specific genes are haploinsufficient will make it feasible to flank each one as closely as possible with pairs of deletions, or to delete these genes in the presence of duplications or transgenic rescue constructs. Further improvements in deletion coverage will undoubtedly identify and map the remaining haplolethal or haplosterile loci. Collectively, our inventories of the RP genes and Minute loci of D. melanogaster provide a solid foundation for further studies of RPs, ribosomes, and the causes and consequences of haploinsufficiency in flies and other organisms. Materials and methods Bioinformatics RefSeq human RP sequences were obtained from the National Center for Biotechnology Information [139]. The FlyBase BLASTp service [140] was used to identify high scoring hits from among the annotated proteins of D. melanogaster; tBLASTn was used when orthologs were not identified by a BLASTp search. The ExPASy proteomics server [141] was used to compute the average pI and molecular weight of the RPs. The percentage identity between human and D. melanogaster RP sequences or between D. melanogaster RP pairs was calculated using the NPS@ ClustalW alignment tool at the Pôle Bioinformatique Lyonnais using default parameters [142,143]. K A /K S values were estimated using the program package PAML [144]. cDNA clone data were obtained from FlyBase [60]. The identification of CRP gene orthologs and the plotting of their evolutionary emergence (Figure 1) were achieved using a combinatorial approach. First, sequences corresponding to the relevant CRP genes from D. melanogaster and Homo sapiens were used as queries in BLASTn, BLASTp, tBLASTn and BLAT searches of the genomes of other Drosophilid and insect species using the FlyBase BLAST server [140] and the UCSC Genome Bioinformatics BLAT server [145,146]. High scoring matches were judged to be potential orthologs and were analyzed further using the FlyBase OrthoView tool [87]. Second, the coding sequences (CDS) of relevant CRP genes from D. melanogaster and H. sapiens were used as queries in BLASTn searches of the GLEANR CDS prediction sets of other Drosophilid species [140]; phylogenetic trees were then generated from the high scoring matches, with the CDS of S. cerevisisae CRPs as roots, using the ClustalW tools at EMBL-EBI [147]. Third, NCBI Homologene [148] was searched for any relevant homology calls: RpLP0-like, RpL7-like and RpL24-like were found in HomoloGene clusters 102093, 64526 and 9462, respectively. The results of all these analyses were then compared, with the most parsimonious interpretations being used to annotate the dendrogram shown in Figure 1. Assessing Minute phenotypes and mapping Minute mutations In order to compile a list of all genetically defined Minute loci, we first catalogued all the Minute loci described in the fly literature [2,54,55,60]. We then inspected deletions for all genomic regions having deletion coverage to confirm or refute the existence of these Minute loci and to identify any new Minute loci that had previously gone undetected. Minute phenotypes were scored primarily by visual inspection of bristle length, although body size, developmental timing, fertility and viability were considered when information was available. Deletion-bearing flies were outcrossed to Oregon-R or Canton-S wild-type strains whenever we could not unambiguously score Minute phenotypes in stocks. The phenotypic effects of deleting or disrupting X-linked genes were assessed only in heterozygous females. Finally, the cytological locations of all verified Minute loci were correlated with the positions of RP genes to identify candidate genes. To assess RP gene haploinsufficiency directly, we inspected flies heterozygous for deletions and/or mutations of molecularly identified RP genes. Minute phenotypes were scored as described above. For some deletions that had not been characterized molecularly, it was necessary to refine the mapping of breakpoints with complementation tests against molecularly mapped mutations or with polytene chromosome preparations to determine whether RP genes were deleted. (In a few cases, RP genes were classified as lacking deletion coverage when the only existing deletions were not useful in a practical sense owing to their associated chromosomal rearrangements or extremely large size.) A transposable element insertion was judged to disrupt a RP gene if it failed to complement other mutations in the gene, if we saw a Minute phenotype in the insertion strain, or if the transposon is inserted in the protein-coding region or 5' UTR of the gene based on FlyBase annotations [87] or our own BLAST analyses. (By these criteria, many nearby insertions, intronic insertions, and insertions in 3' UTRs were not used in our analysis.) We included molecularly characterized point mutations in our analyses for the few RP genes where they were available. Molecularly uncharacterized Minute point mutations from the Bloomington Stock Center were complementation tested against mutations and deletions known to disrupt or delete specific RP genes. Fly strains were obtained from the Bloomington, Szeged, Kyoto and Harvard Drosophila Stock Centers. Helene Doerflinger and Daniel St Johnston provided Df(3R)IR16 and Df(3R)MR22 stocks, and Yuri Sedkov and Alexander Mazo provided mRpL16 A , mRpL16 B and mRpL16 C stocks. Abbreviations CDS, coding sequence; CRP, cytoplasmic ribosomal protein; MRP, mitochondrial ribosomal protein; RNAi, RNA interference; RP, ribosomal protein; rRNA, ribosomal RNA; snoRNA, small nucleolar RNA; UTR, untranslated region. Authors' contributions SJM, JR, GR, AL and KRC conceived, designed and performed the experiments. SJM, MA, PH, ZY and KRC analyzed the data. JR, GR and KRC contributed fly strains. MA, GM and NK helped with gene classification and nomenclature. TCK and SJL provided funds, lab space and general support. SJM and KRC wrote the paper. Additional data files The following additional data are available with the online version of this paper. Additional data file 1 is a table comparing the physical characteristics of D. melanogaster and human CRPs, together with their RefSeq accession numbers. Additional data file 2 is a similar table comparing D. melanogaster and human MRPs. Additional data file 3 is a table listing all the Minute loci in a historical context. Additional data file 4 is a table showing our comprehensive genetic analyses of ribosomal protein gene haploinsufficiency. Additional data file 5 is a table listing CRP gene-Minute locus correspondences arranged in alpha-numerical order by RP gene symbol. Additional data file 6 is a table showing our genetic analyses of translation factor gene haploinsufficiency. Supplementary Material Additional File 1 Comparison of the physical characteristics of D. melanogaster and human CRPs, together with their RefSeq accession numbers. Click here for file Additional File 2 Comparison of the physical characteristics of D. melanogaster and human MRPs, together with their RefSeq accession numbers. Click here for file Additional File 3 Listing of all the Minute loci in a historical context. Click here for file Additional File 4 Comprehensive genetic analyses of ribosomal protein gene haploinsufficiency. Click here for file Additional File 5 CRP gene-Minute locus correspondences arranged in alpha-numerical order by RP gene symbol. Click here for file Additional File 6 Genetic analyses of translation factor gene haploinsufficiency. Click here for file