- Record: found
- Abstract: found
- Article: found
Diagnostic value of HPV E6/E7 mRNA in screening for cervical intraepithelial neoplasia grade 2 or worse: A systematic review and meta‑analysis
Read this article at
Abstract
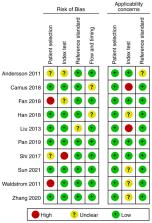
Histology is considered the gold standard for diagnosing the pathological progress of cervical cancer development, while cervical intraepithelial neoplasia of grade 2 or worse (CIN2+) is the cutoff for intervention in clinical practice. The diagnostic value of human papillomavirus (HPV) E6/E7 mRNA in screening for CIN2+ has not been systematically summarized. A meta-analysis was conducted as part of the present study conducted to explore the diagnostic value of HPV E6/E7 mRNA in screening for CIN2+, aiming to provide a new marker for earlier clinical diagnosis of cervical cancer. The PubMed, Embase and Cochrane Library databases were searched from inception to May 2023. Studies reporting the true positive, false positive, true negative and false negative values in differentiating between CIN2+ and CIN2- were included, while duplicate publications, studies without full text, incomplete information or inability to conduct data extraction, animal experiments, reviews and systematic reviews were excluded. STATA software was used to analyze the data. A total of 2,224 patients were included of whom there were 1,274 patients with CIN2+ and 950 patients with CIN2-. The pooled sensitivity and specificity of the studies overall were 0.89 (95% CI, 0.84–0.92) and 0.59 (95% CI, 0.46–0.71), respectively; the positive likelihood ratio (LR) and the negative LR of the studies overall were 2.31 (95% CI, 1.61–3.32) and 0.21 (95% CI, 0.14–0.30), respectively. The pooled diagnostic odds ratio of the studies overall was 11.53 (95% CI, 6.85–19.36). Additionally, the area under the curve was 0.88. The analysis indicated that HPV E6/E7 mRNA has high diagnostic efficacy for CIN2+. HPV E6/E7 mRNA is highly sensitive in the diagnosis of CIN2+, which helps to reduce the rate of missed diagnoses. However, lower specificity may lead to a higher number of misdiagnoses in healthy patients.
Related collections
Most cited references29
- Record: found
- Abstract: not found
- Article: not found
Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.
- Record: found
- Abstract: found
- Article: not found
Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older.
- Record: found
- Abstract: found
- Article: not found