- Record: found
- Abstract: found
- Article: found
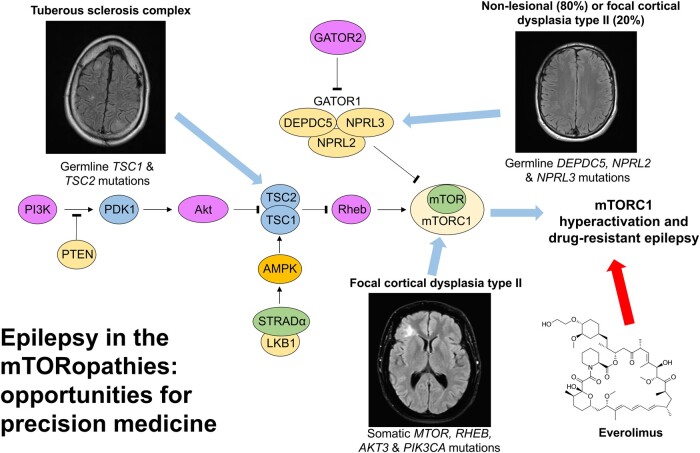
Epilepsy in the mTORopathies: opportunities for precision medicine

Read this article at
Abstract
The mechanistic target of rapamycin signalling pathway serves as a ubiquitous regulator of cell metabolism, growth, proliferation and survival. The main cellular activity of the mechanistic target of rapamycin cascade funnels through mechanistic target of rapamycin complex 1, which is inhibited by rapamycin, a macrolide compound produced by the bacterium Streptomyces hygroscopicus. Pathogenic variants in genes encoding upstream regulators of mechanistic target of rapamycin complex 1 cause epilepsies and neurodevelopmental disorders. Tuberous sclerosis complex is a multisystem disorder caused by mutations in mechanistic target of rapamycin regulators TSC1 or TSC2, with prominent neurological manifestations including epilepsy, focal cortical dysplasia and neuropsychiatric disorders. Focal cortical dysplasia type II results from somatic brain mutations in mechanistic target of rapamycin pathway activators MTOR, AKT3, PIK3CA and RHEB and is a major cause of drug-resistant epilepsy. DEPDC5, NPRL2 and NPRL3 code for subunits of the GTPase-activating protein (GAP) activity towards Rags 1 complex (GATOR1), the principal amino acid-sensing regulator of mechanistic target of rapamycin complex 1. Germline pathogenic variants in GATOR1 genes cause non-lesional focal epilepsies and epilepsies associated with malformations of cortical development. Collectively, the mTORopathies are characterized by excessive mechanistic target of rapamycin pathway activation and drug-resistant epilepsy. In the first large-scale precision medicine trial in a genetically mediated epilepsy, everolimus (a synthetic analogue of rapamycin) was effective at reducing seizure frequency in people with tuberous sclerosis complex. Rapamycin reduced seizures in rodent models of DEPDC5-related epilepsy and focal cortical dysplasia type II. This review outlines a personalized medicine approach to the management of epilepsies in the mTORopathies. We advocate for early diagnostic sequencing of mechanistic target of rapamycin pathway genes in drug-resistant epilepsy, as identification of a pathogenic variant may point to an occult dysplasia in apparently non-lesional epilepsy or may uncover important prognostic information including, an increased risk of sudden unexpected death in epilepsy in the GATORopathies or favourable epilepsy surgery outcomes in focal cortical dysplasia type II due to somatic brain mutations. Lastly, we discuss the potential therapeutic application of mechanistic target of rapamycin inhibitors for drug-resistant seizures in GATOR1-related epilepsies and focal cortical dysplasia type II.
Abstract
Moloney et al. outline the spectrum of epilepsies caused by pathogenic variation in genes encoding for regulators of the mechanistic target of rapamycin signalling pathway. Epileptogenesis in the mTORopathies results from excessive activation of the pathway, presenting a therapeutic target for seizures and other manifestations in these disorders.
Graphical Abstract
Related collections
Most cited references170
- Record: found
- Abstract: found
- Article: not found
mTOR signaling in growth control and disease.
- Record: found
- Abstract: found
- Article: not found
Mutation and cancer: statistical study of retinoblastoma.
- Record: found
- Abstract: found
- Article: not found