- Record: found
- Abstract: found
- Article: found
Mechanisms of Parkinson’s disease-related proteins in mediating secondary brain damage after cerebral ischemia
Read this article at
Abstract
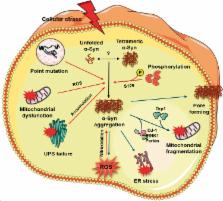
Both Parkinson’s disease (PD) and stroke are debilitating conditions that result in neuronal death and loss of neurological functions. These two conditions predominantly affect aging populations with the deterioration of the quality of life for the patients themselves and a tremendous burden to families. While the neurodegeneration and symptomology of PD develop chronically over the years, post-stroke neuronal death and dysfunction develop rapidly in days. Despite the discrepancy in the pathophysiological time frame and severity, both conditions share common molecular mechanisms that include oxidative stress, mitochondrial dysfunction, inflammation, endoplasmic reticulum stress, and activation of various cell death pathways (apoptosis/necrosis/autophagy) that synergistically modulate the neuronal death. Emerging evidence indicates that several proteins associated with early-onset familial PD play critical roles in mediating the neuronal death. Importantly, mutations in the genes encoding Parkin, PTEN-induced putative kinase 1 and DJ-1 mediate autosomal recessive forms of PD, whereas mutations in the genes encoding leucine-rich repeat kinase 2 and α-synuclein are responsible for autosomal dominant PD. This review discusses the significance of these proteins with the emphasis on the role of α-synuclein in mediating post-ischemic brain damage.
Related collections
Most cited references147
- Record: found
- Abstract: found
- Article: not found
Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases.
- Record: found
- Abstract: found
- Article: not found
alpha-Synuclein is phosphorylated in synucleinopathy lesions.
- Record: found
- Abstract: found
- Article: not found