- Record: found
- Abstract: found
- Article: not found
Effect of 1,3-Propane Sultone on the Formation of Solid Electrolyte Interphase at Li-Ion Battery Anode Surface: A First-Principles Study
Read this article at
Abstract
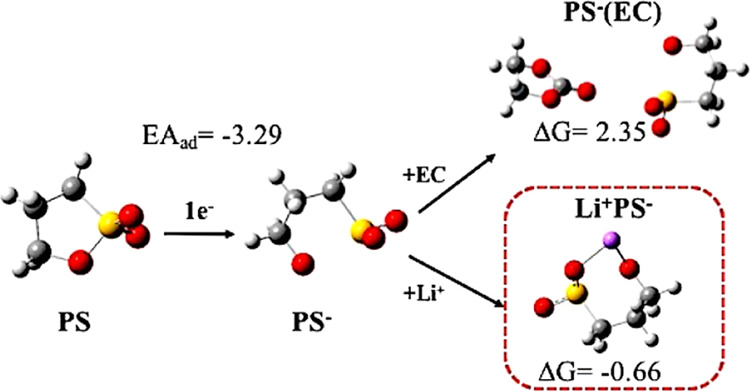
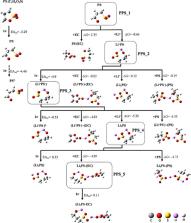
Density functional theory is applied to investigate the reductive reactions of reductive-type additive, 1,3-propane sultone (PS), on the formation of solid electrolyte interphase (SEI) near the lithium-ion battery anode surface. Different from the studies that mostly focus on the reduction dissociation of a specific molecule, we adopt an iterative method that systematically considered most possible reactants from the environment in every round of the reaction. The thermodynamically favorable reaction in each round was chosen. Its products then proceed to the following step. At least four iterations of reactions were calculated. The favorable products in each round were then analyzed to understand the trend of the series reactions. With the iterative method, the compounds in every reaction round can be inspected in detail. The method not only predicted the compounds that are consistent with those observed in the experiments but also provide insights into how PS forms an effective SEI. In the solvent state, the most stable reduction states of PS and electrolyte ethylene carbonate (EC) are confirmed as the initial reactants further interact with the environment supplies. First, with the addition of PS, the reduction of PS is prior to EC, which would suppress the reduction of EC and decrease the generation of ethene gas. Second, the compounds from the initial reaction round of PS are lithiated ones and show higher reduction ability than that of EC, while the latter show lower reduction ability than that of the EC, which terminated the reactions. This would be the critical properties for reductive-type additive to form an effective SEI film.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model.
- Record: found
- Abstract: not found
- Article: not found
The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model
- Record: found
- Abstract: not found
- Article: not found
