- Record: found
- Abstract: found
- Article: found
Associations Between Dietary Protein Sources, Plasma BCAA and Short-Chain Acylcarnitine Levels in Adults
Read this article at
Abstract
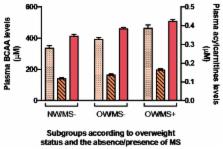
Elevated plasma branched-chain amino acids (BCAA) and C3 and C5 acylcarnitines (AC) levels observed in individuals with insulin resistance (IR) might be influenced by dietary protein intakes. This study explores the associations between dietary protein sources, plasma BCAA levels and C3 and C5 ACs in normal weight (NW) or overweight (OW) individuals with or without metabolic syndrome (MS). Data from 199 men and women aged 18–55 years with complete metabolite profile were analyzed. Associations between metabolic parameters, protein sources, plasma BCAA and AC levels were tested. OW/MS+ consumed significantly more animal protein ( p = 0.0388) and had higher plasma BCAA levels ( p < 0.0001) than OW/MS− or NW/MS− individuals. Plasma BCAA levels were not associated with BCAA intakes in the whole cohort, while there was a trend for an association between plasma BCAA levels and red meat or with animal protein in OW/MS+. These associations were of weak magnitude. In NW/MS− individuals, the protein sources associated with BCAA levels varied greatly with adjustment for confounders. Plasma C3 and C5 ACs were associated with plasma BCAA levels in the whole cohort ( p < 0.0001) and in subgroups based on OW and MS status. These results suggest a modest association of meat or animal protein intakes and an association of C3 and C5 ACs with plasma BCAA levels, obesity and MS.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Dietary factors and low-grade inflammation in relation to overweight and obesity.
- Record: found
- Abstract: found
- Article: not found
Amino acid signalling upstream of mTOR.
- Record: found
- Abstract: found
- Article: not found