- Record: found
- Abstract: found
- Article: found
Daratumumab combined with dexamethasone and lenalidomide or bortezomib in relapsed/refractory multiple myeloma (RRMM) patients: Report from the multiple myeloma GIMEMA Lazio group
Read this article at
Abstract
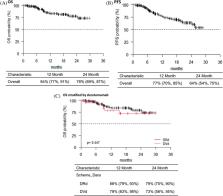
The multiple myeloma (MM) treatment has changed over the last years due to the introduction of novel drugs. Despite improvements in the MM outcome, MM remains an incurable disease. Daratumumab is a human IgGK monoclonal antibody targeting CD38 with tumor activity associated with immunomodulatory mechanism. In combination with standard of care regimens, including bortezomib (Vd) or lenalidomide (Rd), daratumumab prolonged progression‐free survival (PFS) in patients (pts) with relapsed/refractory multiple myeloma (RRMM) and in new diagnosis MM. We report the data of the MM GIMEMA Lazio group in 171 heavily treated pts who received daratumumab, lenalidomide and dexamethasone (DRd) or daratumumab, velcade and dexamethasone (DVd). The overall response rate was 80%, and the overall survival (OS) and PFS were 84% and 77%, respectively. In addition, pts treated with DRd showed a better median PFS compared to pts treated with DVd, at 12 and 24 months, respectively. The most common hematologic treatment‐emergent adverse events (TAEs) were neutropenia, thrombocytopenia, and anemia. The most common nonhematologic TAEs were peripheral sensory neuropathy and infections. Our data confirmed that DRd or DVd therapy is effective and safe in RRMM pts, and our real‐life analysis could support the physicians regarding the choice of optimal therapy in this setting of pts.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support.
- Record: found
- Abstract: found
- Article: not found
The REDCap consortium: Building an international community of software platform partners
- Record: found
- Abstract: found
- Article: not found