- Record: found
- Abstract: found
- Article: found
Are we following the guidelines on non-muscle invasive bladder cancer?
Read this article at
ABSTRACT
Objectives
To evaluate the clinical practice of non-muscle invasive bladder cancer (NMIBC) treatment in Brazil in relation to international guidelines: Sociedade Brasileira de Urologia (SBU), European Association of Urology (EAU) and American Urological Association (AUA).
Materials and Methods
Cross-sectional study using questionnaires about urological practice on treatment of NMIBC during the 32nd Brazilian Congress of Urology. A total of 650 question forms were answered.
Results
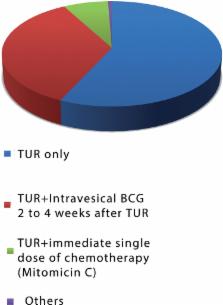
There were 73% of complete answers (total of 476 question forms). In total, 246 urologists (51.68%) lived in the southeast region and 310 (65.13%) treat 1 to 3 cases of NMIBC per month. Low risk cancer: Only 35 urologists (7.5%) apply the single intravesical dose of immediate chemotherapy with Mitomicin C recommended by the above guidelines. Adjuvant therapy with BCG 2 to 4 weeks after TUR is used by 167 participants (35.1%) and 271 urologists (56.9%) use only TUR. High risk tumors: 397 urologists (83.4%) use adjuvant therapy, 375 (78.8%) use BCG 2 to 4 weeks after TUR, of which 306 (64.3%) referred the use for at least one year. Intravesical chemotherapy with Mitomicin C (a controversial recommendation) was used by 22 urologists (4.6%). BCG dose raised a lot of discrepancies. Induction doses of 40, 80 and 120mg were referred by 105 (22%), 193 (40.4%) and 54 (11.3%) respectively. Maintenance doses of 40, 80 and 120mg were referred by 190 (48.7%), 144 (37.0%) and 32 (8.2%) urologists, respectively. Schemes of administration were also varied and the one cited by SWOG protocol was the most used: 142 (29.8%).
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study.
- Record: found
- Abstract: not found
- Article: not found
Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update.
- Record: found
- Abstract: found
- Article: not found