- Record: found
- Abstract: found
- Article: found
Piezo1 Channels Contribute to the Regulation of Human Atrial Fibroblast Mechanical Properties and Matrix Stiffness Sensing
Read this article at
Abstract
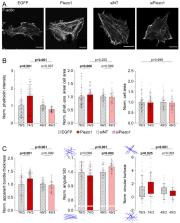
The mechanical environment of cardiac cells changes continuously and undergoes major alterations during diseases. Most cardiac diseases, including atrial fibrillation, are accompanied by fibrosis which can impair both electrical and mechanical function of the heart. A key characteristic of fibrotic tissue is excessive accumulation of extracellular matrix, leading to increased tissue stiffness. Cells are known to respond to changes in their mechanical environment, but the molecular mechanisms underlying this ability are incompletely understood. We used cell culture systems and hydrogels with tunable stiffness, combined with advanced biophysical and imaging techniques, to elucidate the roles of the stretch-activated channel Piezo1 in human atrial fibroblast mechano-sensing. Changing the expression level of Piezo1 revealed that this mechano-sensor contributes to the organization of the cytoskeleton, affecting mechanical properties of human embryonic kidney cells and human atrial fibroblasts. Our results suggest that this response is independent of Piezo1-mediated ion conduction at the plasma membrane, and mediated in part by components of the integrin pathway. Further, we show that Piezo1 is instrumental for fibroblast adaptation to changes in matrix stiffness, and that Piezo1-induced cell stiffening is transmitted in a paracrine manner to other cells by a signaling mechanism requiring interleukin-6. Piezo1 may be a new candidate for targeted interference with cardiac fibroblast function.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Fiji: an open-source platform for biological-image analysis.
- Record: found
- Abstract: found
- Article: not found
Matrix elasticity directs stem cell lineage specification.
- Record: found
- Abstract: found
- Article: not found